Cerebral regional oxygen saturation monitoring in pediatric malfunctioning shunt patients
 Cerebral regional oxygen saturation moni”>American Journal of Emergency Medicine (2013) 31, 365-374
Cerebral regional oxygen saturation moni”>American Journal of Emergency Medicine (2013) 31, 365-374

Original Contribution
Cerebral regional oxygen saturation monitoring in pediatric malfunctioning shunt patients?,??,?
Thomas J. Abramo MD a,?, Chuan Zhou PhD c, Cristina Estrada MD a,
Patrick C. Drayna MD a, Matthew R. Locklair MD a, Renee Miller RN, MSN a,
Matthew Pearson MD b, Noel Tulipan MD b, Donald H. Arnold MD, MPH a
aDepartment of Pediatrics, Division of Pediatric Emergency Medicine, Vanderbilt University School of Medicine, Nashville, TN bDepartment of neurosurgery, Division of Pediatric Neurosurgery, Vanderbilt University School of Medicine, Nashville, TN cCenter for Child Health, Behavior, and Development, Seattle Children’s Research Institute, Department of Pediatrics, University of Washington School of Medicine, Seattle, WA
Received 19 June 2012; revised 5 September 2012; accepted 5 September 2012
Abstract
Background: Shunt malfunction produces increased intracranial pressure causing decreased cerebral regional perfusion and tissue O2sat. Cerebral regional oxygen saturation (rSO2) by Near-infrared spectroscopy represents tissue perfusion and oxygen saturation. Cerebral rSO2 is used to detect cerebral ischemia in pediatric clinical settings.
Objective: The objective of the study was to determine the reliability of cerebral rSO2 in pediatric malfunctioning shunt.
Methods: A prospective observational study of pediatric patients presented to the pediatric emergency department was conducted. Confirmed malfunctioning shunt subjects had cerebral rSO2 monitoring. Results: A total of 131 malfunctioning shunt subjects had cerebral rSO2 monitoring. Patient’s central trend and intrasubject variability of cerebral rSO2 readings for left and right probe and malfunction sites (n = 131) are as follows:
|
Variable
|
Overall, mean SO2 |
Distal, mean SO2 |
Proximal, mean rSO2 |
P |
|
|
Left cerebral rSO2 trend |
69.1 (10.7) |
67.7 (9.81) |
70.0 |
(11.17) |
.23 |
|
Right cerebral rSO2 trend |
71.3 (9.6) |
70.5 (8.13) |
71.8 |
(10.40) |
.42 |
|
Left cerebral rSO2 variability |
3.57 (2.04) |
4.72 (2.55) |
2.88 |
(1.24) |
b.001 |
|
Right cerebral rSO2 variability |
3.46 (1.95) |
3.77 (2.20) |
3.28 |
(1.77) |
.19 |
? Financial support: Internal funding support was provided by the Vanderbilt Institute for Clinical and Translational Research. Somanetics Corporation provided near-infrared machines and a limited number of near-infrared spectroscopy probes used in the study. Company representatives otherwise had no input into the design, execution, data analysis, or preparation of this manuscript.
?? The authors have no conflicts of interest to disclose.
? Thomas Abramo and Cristina Estrada wrote the first draft of the manuscript, and no honorarium, grant, or other form of payment was given to anyone to
produce the manuscript.
* Corresponding author. Department of Pediatrics, Division of Pediatric Emergency Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232-9001. Tel.: +1 615 936 3898; fax: +1 615 322 4374.
E-mail address: [email protected] (T.J. Abramo).
0735-6757/$ – see front matter (C) 2013 http://dx.doi.org/10.1016/j.ajem.2012.09.006
Intrasubject left and right rSO2 Pearson correlation was -0.46 to 0.98 (mean +- SD, 0.35 +- 0.34; median, 0.34; interquartile range, 0.06-0.61). The Correlation coefficients of 99 subjects between left and right rSO2 was significantly different (P b .001), suggesting that intrasubjects’ left and right rSO2 are highly correlated. Sample mean difference between left and right rSO2 were -1.7% (95% confidence interval [CI], -1.8 to -1.6; P b .001) supporting overall left lower than right. Intraclass correlation for left rSO2 was 87.4% (95% CI, 87.2%-87.6%), and that for right rSO2 was 83.8% (95% CI, 83.8%-84%), showing intersubject differences accounting for the variation, and relative to intersubject variation, intrasubjects readings are consistent. Intrasubjects, left and right rSO2 highly correlate and are asymmetrical. Left and right rSO2 are consistent in intrasubject with large rSO2 variations in trend and variability across subjects.
Conclusion: This study demonstrates reliable cerebral rSO2 readings in subjects with malfunctioning shunts, with asymmetrical cerebral rSO2 hemispheric dynamics within subjects.
(C) 2013
Introduction
Hydrocephalus is a disorder caused by a disruption in the normal transport of cerebral spinal fluid (CSF). Disrupted CSF flow results in an accumulation of fluid leading to increased intracranial pressure , altered cerebrovascular autoregulation, and a decrease in brain perfusion [1,2]. In most cases, placement of an extracranial shunt presents the best treatment option.
A recent study estimated that 33 000 pediatric shunt procedures are performed every year within the United States. The same study also estimated that approximately 5% to 80% of patients will experience at least 1 episode of shunt malfunction in the first 10 years after insertion, and the annual rate of shunt malfunction is estimated to be 5% to 25%, with most of these patients presenting to an emergency department [2-8].
Shunt malfunction results in increased fluid collection producing increased ICP [2-6]. This rise in ICP may decrease cerebral blood flow, regional perfusion, and oxygen delivery, promoting anaerobic metabolism and impairing cerebral tissue oxygen. Depending on the severity of hydrocephalus, type of shunt, cognitive/neurologic develop- ment of the child, and duration of the increase in ICP, signs and symptoms can be subtle or pronounced [3-8]. Most of these patients present to an emergency department with headache, nausea, vomiting, drowsiness, somnolence, irrita- bility, seizures, and other signs of increased ICP [3-8]. However, many symptoms occur in the context of other common pediatric illnesses, making it very difficult to distinguish patients with a shunt malfunction from those without. Given the associated high morbidity and mortality of shunt malfunction, early detection is of critical impor- tance. Standard emergency department evaluation of sus- pected ventricular shunt malfunction consists of plain radiographs of the skull, neck, chest, and abdomen (shunt series), to determine the presence of mechanical breaks, kinks, and disconnections in the shunt tubing [4,9,10]. Computed tomography (CT) of the head is then obtained to detect a change in ventricular size [4,9,10]. This method of
evaluation is very labor intense and time-consuming, and radiographs and CT scans expose children to Large doses of repeated radiation [9,10]. There is significant concern that CT scans expose children to potentially harmful radiation doses [11-13]. Studies have demonstrated the benefit of fast magnetic resonance imaging to evaluate ventricular size in patients with suspected shunt malfunction detection, but this evaluation method is more expensive than CT scan and in limited use. In addition, studies involving serum biomarkers for rapid assessment for shunt malfunction have showed no significant promise in identifying shunt malfunctions. Overall, the current standard of care has not changed significantly in more than 40 years.
Near-infrared spectrometry (NIRS) absorption data reflect a balance between oxygenated hemoglobin and deoxyhe- moglobin present in the tissue, from which regional oxygenation (rSO2) percentage values are calculated [11,12]. Values of rSO2 represent tissue perfusion (capillary integrity, local blood volume, local vasomotor tone, and perfusion pressure), oxygen availability (oxygenate hemo- globin [O2Hb], total hemoglobin), and overall tissue metabolism [2,13]. Most of tissue hemoglobin is in the venous circulation; NIRS gives a venous-weighted relative oxygen index of tissue beneath the probe placement (Fig. 1). On cerebral and/or somatic rSO2 readings, clinicians can infer metabolic changes in the regional tissue’s oxygen supply, Oxygen extraction, and tissue metabolism. Cerebral and/or somatic near-infrared spectroscopy can more quickly and noninvasively identify patients with altered levels of cerebral and/or somatic Tissue oxygenation and, in conjunc- tion with global physiologic parameters, guide efficient and effective resuscitation to improve outcomes for critically ill and injured pediatric patients in various clinical situations (neuroprotection, adult and pediatric heart surgery, trauma, compartment syndrome, adult cardiac arrest/cardiopulmo- nary resuscitation, traumatic brain injury, pediatric diabetic ketoacidosis) [13,14,2,15-17]. Cerebral and somatic rSO2 by NIRS monitoring can detect clinically silent episodes of cerebral and/or somatic ischemia in a variety of clinical settings and provide a safeguard for cerebral and/or somatic

Fig. 1 Cerebral rSO2 Near Infrared Spectroscopy sampling process.
function [13,14,2,15-17]. The most common application is in intraoperative neurophysiologic monitoring in children who undergo repair of congenital cardiac disease, critically ill neonates, and pediatric patients with traumatic brain injury [13,16]. Near-infrared spectrometry monitoring system is noninvasive, is easy to apply, gives instantaneous readings, and prompts early interventions to maintain hemodynamic stability of the patient, resulting in decreased rates of neurologic and somatic complications [16-18].
During episodes of shunt malfunction, increased ICP results in changes in brain tissue perfusion, metabolism, and oxygen extraction [15-18]. Cerebral rSO2 monitoring may have a unique capability of detecting these subtle changes. Near-infrared cerebral monitoring studies have demonstrated that rSO2 monitoring is an effective noninvasive technique for detecting real-time cerebrovascular and oxygenation changes in the critically ill and injured child with normal cerebral anatomical structure [13,15-19]. Although exten- sive data exist demonstrating the effectiveness of cerebral rSO2 NIRS as a diagnostic technique in those settings, no studies to date have evaluated the effects of increased ICP due to malfunctioning shunts in pediatric subjects with abnormal cerebral anatomical structure on the reliability of cerebral rSO2 readings.
In our prior investigations, we have demonstrated reliable cerebral rSO2 continuous readings with high intersubject and
low intrasubject variability in patients with hydrocephalus who have functioning shunts [20]. In the pediatric mal- functioning shunts, the increased ICP effects on cerebral perfusion and tissue oxygenation as demonstrated by rSO2 NIRS in the abnormal brain structure has not been published; therefore, consistent and reliable continuous cerebral rSO2 readings will be hard to predict individually or in a group compared with their functioning shunts. We are not aware of any studies examining the reliability of continuous cerebral rSO2 in assessing cerebral perfusion in pediatric patients during ventricular shunt malfunction. Our primary objective was to conduct a descriptive study on determining the reliability of continuous cerebral rSO2 readings in pediatric patients with hydrocephalus who have malfunctioning ventricular shunts in an emergency setting. Not monitoring the subjects when their shunts were functioning afterward was not the primary intent of this study.
Methods
We conducted a prospective observational study of pediatric patients with a history of hydrocephalus who had at least one ventricular shunt in place and presented to the pediatric emergency department (PED) between March 2007
and July 2010, with a concern for shunt malfunction. Pediatric emergency department patients with signs or symptoms of shunt malfunction had routine evaluation of their shunt, including a CT scan of the head and shunt series (x-rays of the head/neck, chest, and abdomen). Subjects were enrolled only if they had shunt malfunction confirmed by the attending radiologist and neurosurgery’s interpretation of these studies. Parental consent and child assent (when appropriate) were obtained after radiologic confirmation for malfunctioning shunt. Subjects were excluded if they had fever or other indicators for possible infected shunt, fever with shunt revision less than 3 months ago, or abscess/ cellulitis overlying the shunt. Subjects were excluded if structural abnormalities (example scaring) of the forehead prevented the application of the NIRS probes. Once subjects were consented, continuous monitoring of their rSO2 values was accomplished by using the portable Somanetics INVOS 5100C Cerebral/Somatic Near Infrared Spectroscopy Oxim- eter (Troy, MI), which captures cerebral or somatic regional tissue venous oxygenation readings every 5 seconds and displays the readings as a percentage of oxygenated regional hemoglobin. Upon placement of the NIRS probes, there are instantaneous digital and trend graphs for each probe. Near- infrared spectrometry probes were placed on the subject’s left and right forehead and remained in the original placement site during the PED clinical evaluation. The probes were removed before transfer to the floor or pediatric critical care unit or the operating room. Patient’s operative notes were reviewed for the definitive type of malfunctioning shunt, distal vs proximal. Near-infrared spectrometry monitoring did not compromise Clinical decisions, diagnostic tests, or Therapeutic measures administered by the treating health care providers. The primary objective of the study protocol was to determine the reliability of continuous cerebral rSO2 readings in altered cerebral tissue because of the malfunctioning shunt; further cerebral rSO2 monitoring during diagnostic testing was not the objective. The study protocol was reviewed and approved by the institutional review board.
Statistical analysis
CT characteristics“>Subject characteristics were summarized with descriptive statistics. Cerebral rSO2 analysis was generated by left or right probe readings not by shunt side (left and right generated cerebral analysis) due low number of left side shunts owing to the standard of practice of shunt placement, are on the right cerebral hemisphere. Categorical variables were summarized using frequency and proportions. Contin- uous variables were summarized with means and SDs.
Continuous cerebral rSO2 by NIRS monitoring generated consecutive multiple readings per subject from both left and right probes placed on subject’s forehead. For each subject and each probe, we estimated the central trend of rSO2 using the mean of rSO2 readings and the variability of rSO2 using the SD of cerebral rSO2 readings. This procedure generated
4 additional subject-level data points, corresponding to trend and variability in rSO2 from each side of probes. Sample level summary statistics were then calculated based on these data. To assess the within-patient correlation between cerebral rSO2 readings from left and right probes, we calculated the Pearson correlation coefficient for cerebral rSO2 readings from probes on both sides. The proportion of subjects with significant correlations between left and right probes was reported.
We applied multiple procedures to examine the agreement between cerebral rSO2 readings from left and right probes. First, we examined whether there was a systematic difference between cerebral rSO2 readings from left and right probes by comparing readings from both sides for each subject. We also tested the overall difference between the 2 sides. Student t test was used for comparisons. Second, we used Bland- Altman plots to assess the agreement between left and right probes in terms of rSO2 trend and variability. Third, we examined whether trend and variability were associated with the site of shunt malfunction and other patient characteristics using the t test.
To show that the cerebral rSO2 readings were consistent within subjects and also specific to subjects, we would like to show that the cerebral rSO2 readings had small within- subject variations (the readings were similar within a subject), relative to large between-subject variations (the readings were quite different across subjects). Toward this aim, we applied repeated analysis of variance model to cerebral rSO2 readings from each side, respectively. The contribution of between-subject variation to the total variation was reported as intraclass correlation (ICC) and was reported. Bootstrap method was used to derive the confidence intervals (CIs) on ICCs. Variability among the bilateral NIRS measurements was explored using Bland- Altman analysis of the difference between measurements compared with the average of measurements.
All analyses were conducted using the R statistical package version 2.12.1 [21].
Results
Subject characteristics
Subject characteristics are summarized in Table 1. One hundred thirty-one subjects with confirmed malfunctioning shunts by positive CT scan and/or shunt series were enrolled. No patients refused enrollment into the study. No subjects recruited and consented for the study withdrew from the monitoring period, and no duplicate subject monitoring occurred in the study period. Subjects’ ages ranged from 1 month to 18 years, with an average (SD) of 5.03 (4.5) years. There were 75 (58%) males. Thirty-seven percent of the subjects had seizure history. Most subjects were developmen- tally delayed (69%). Most subjects had shunts placed on the
|
Variables |
Overall |
Malfunction site |
P |
||
|
Distal |
Proximal |
||||
|
n |
131 |
49 (37%) |
82 (63%) |
.005 |
|
|
Age (y) |
5.03 (4.52) |
5.36 (5.10) |
4.83 (4.15) |
.54 |
|
|
Seizure history |
49 (37%) |
20 (41%) |
29 (35%) |
.53 |
|
|
Development delay |
90 (69%) |
37 (76%) |
53 (65%) |
.19 |
|
|
Shunt side |
.74 |
||||
|
Left |
18 (14%) |
8 (16%) |
10 (12%) |
||
|
Right |
111 (85%) |
40 (82%) |
71 (87%) |
||
|
Both |
2 (2%) |
1 (2%) |
1 (1%) |
||
|
Patient’s central trend and within-subject variability of cerebral rSO2 readings for left and right probes and malfunction sites (n = 131)
|
||||
|
Variable |
Overall, mean rSO2 |
Distal, mean SO2 |
Proximal, mean SO2 |
P |
|
Left cerebral rSO2 trend |
69.1 (10.7) |
67.7 (9.81) |
70.0 (11.17) |
.23 |
|
Right cerebral rSO2 trend |
71.3 (9.6) |
70.5 (8.13) |
71.8 (10.40) |
.42 |
|
Left cerebral rSO2 variability |
3.57 (2.04) |
4.72 (2.55) |
2.88 (1.24) |
b.001 |
|
Right cerebral rSO2 variability |
3.46 (1.95) |
3.77 (2.20) |
3.28 (1.77) |
.19 |
right side (85%). Shunt drainage systems were ventriculoper- itoneal in 125 subjects (95.4%), ventricular-atrial in 3 subjects, ventricular-pleural in 2 subjects, and ventricular gall bladder in 1 subject. Shunt malfunction location was more often proximal (n = 82; 63%) than distal (n = 49; 37%).
Table 1 Sample characteristics and central trend and within-subject variability of cerebral rSO2.
Review of the PED and hospital electronic medical records demonstrated that no enrolled subjects withdrew from the study, mean (SD) durations of malfunctioning shunt symptoms were 3.4 (1.1) days, and all had Surgical repair of their shunts. All subjects had shunt revisions, none had shunt infections (no growth on final CSF cultures obtained from the operating room and/or Shunt tap), and 72% received shunt taps. All subjects were admitted to the operating room or to the pediatric critical care unit with subsequent surgical repair. There were no deaths from malfunctioning shunts.
Cerebral rSO2 measurements
The cerebral rSO2 readings from 16 randomly selected subjects are shown in Fig. 2. Cerebral rSO2 levels as measured by cerebral rSO2 show a large amount of variation in cerebral rSO2 readings differed between left and right probes within a subject and between subjects. For many subjects, the cerebral rSO2 readings demonstrated cyclic patterns. Overall, we collected a total of 84 907 readings from all probes (Table 1). The observation numbers per subject ranged from 127 to 4413, with a mean (SD) of 961 (661). For all subsequent analyses, we only considered cerebral rSO2 readings when subjects did not receive shunt taps. For subjects who did receive tap, we used their cerebral rSO2 readings before the shunt tap for the analysis, which was the primary study objective. We only analyzed
pretapped left and right frontotemporal cerebral rSO2 readings in the 131 malfunctioning shunt study subjects.
Evaluation of bilateral cerebral rSO2
For each subject and each different forehead probe location, the central trend was estimated using means and variability using SDs. For probes placed on left forehead, the cerebral rSO2 trend statistic ranged from 40.6% to 94%, with a mean (SD) of 69.1% (10.7%). For probes placed on the right forehead, within-subject cerebral rSO2 trend statistic ranged from 41% to 93.9%, with a mean (SD) of 71.3% (9.6%). The within-subject variability statistic ranged from 1.09% to 12.1%, with a mean (SD) of 3.57% (2.04%) for the left cerebral rSO2, and ranged from 1.11% to 10.5%, with a mean (SD) of 3.46% (1.95%) for the right cerebral rSO2. Distal shunt malfunction is significantly associated with higher variability in cerebral rSO2 from left cerebral rSO2 (Table 1). There was no significant association between shunt malfunc- tion sites and central cerebral rSO2 trends from both channels. For each subject, we calculated the Pearson correlation coefficient between readings from left and right probes. The correlation coefficients ranged from -0.46 to 0.98 (mean +- SD, 0.35 +- 0.34; median, 0.34; interquartile range, 0.06-0.61). Ninety nine (76%) subjects had correlation coefficients between left and right readings significantly different from 0 (P b .001 to account for multiple comparisons), suggesting that within the same subject, cerebral rSO2 values from left and
right readings tend to be highly correlated.
Fig. 3 shows the pairwise differences between left and right cerebral rSO2 for each subject. The points represent mean differences, and the vertical lines correspond to +-1.96 SD. The width of each vertical line shows the variability of
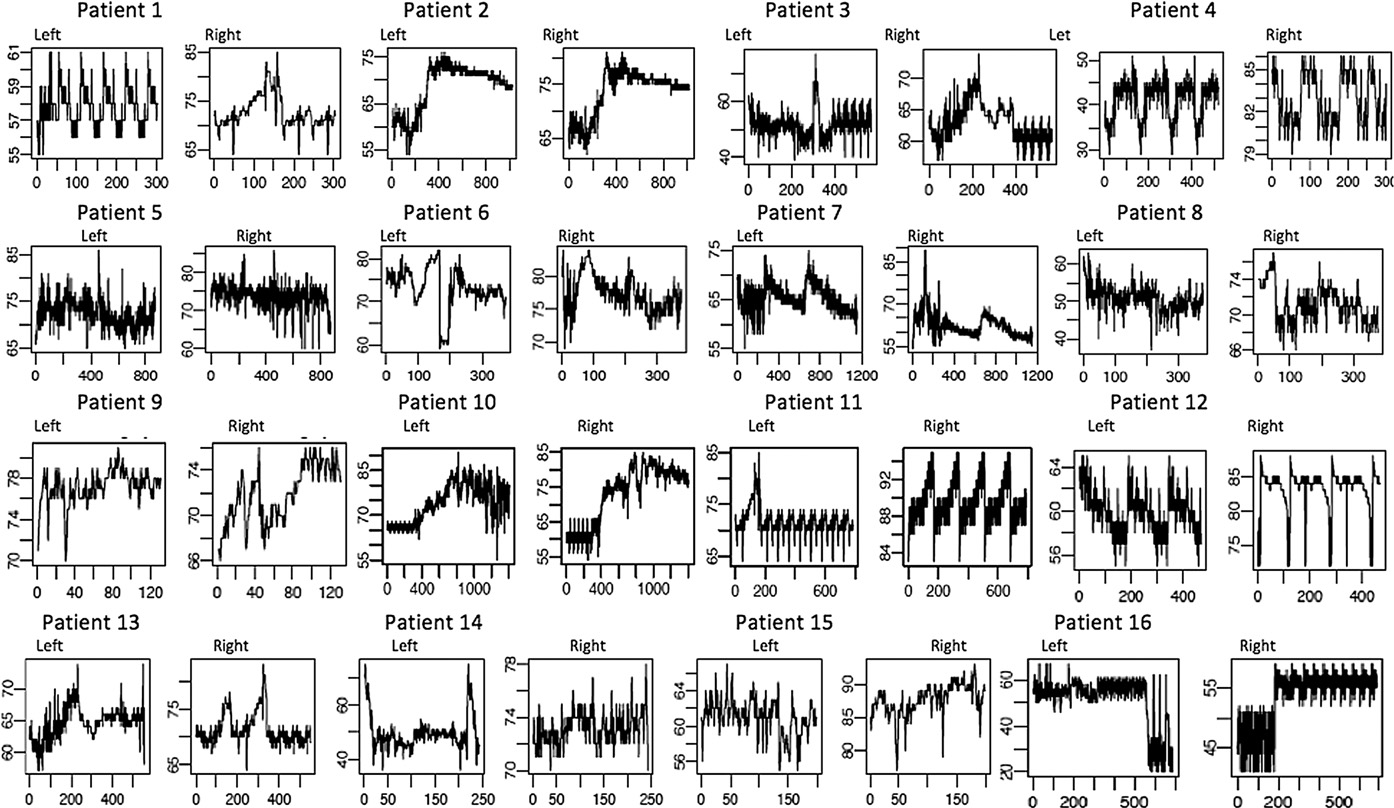
Fig. 2 Malfunctioning Shunt Cerebral rSO2 Time Flow (16 random subject selection (Total N = 131 study subjects): Demonstrating Subject’s Unique & Asymmetrical Cerebral Dynamics: Cerebral regional tissue oxygenation (rSO2) readings in % over time: X axis = rSO2 values as %, Y axis = time in seconds, Left = Left cerebral rSO2 readings, Right = Right cerebral rSO2 readings.
the left-right differences for each patient. Fifty-three (41%) subjects had mean left-right differences greater than zero; that is, on average, left readings are larger than the right readings. Fifty-four (41%) vertical lines do not cover zero, meaning the mean differences are significantly different from 0. The sample mean difference between left and right cerebral rSO2 readings was -1.7% (95% CI, -1.8 to -1.6; P b .001), suggesting that overall left cerebral rSO2 tends to be lower than right. Also noted was the large variability in

Bland-Altman plots for trend and va”>Fig. 3 Pair-wise cerebral rSO2 Differences between Left and Right rSO2 reading for each Subject.
the mean differences and CI width across patients. These results suggest that within individual subjects, cerebral rSO2 readings from left and right probes tend to be highly correlated. However, they are not the same, and there are systematic differences between the 2 cerebral rSO2 readings. intraclass correlation coefficient for left cerebral rSO2 was estimated to be 87.4% (95% CI, 87.2%-87.6%), and that of right cerebral rSO2 was 83.8% (95% CI, 83.6%-84%), suggesting that across-subject differences account for most of the variation in the data, and relative to between-subject variation, the readings within subjects are more consistent. This means that almost 85% of the variability in the data, regardless of cerebral rSO2, is due to differences across subjects. Such large between-subject variability further supports our claim that it is unreasonable to use one group of subjects as controls for another group of subjects. In clinical pediatric cardiac critical care practice, the accept norm for the difference between the interhemispheric cerebral rSO2 is 5% to 10%, but no studies have define
trend and variability in these patients.
Bland-Altman plots for trend and variability
The plots in Fig. 4 show that there lacks agreement in trend between left and right cerebral rSO2 readings. However, among those with smaller overall variability in rSO2 (the points to the left of the vertical line), there is good

Fig. 4 (A) Bland-Altman Plot for cerebral rSO2 Trend between Left and Right cerebral rSO2 (B) Bland-Altman Plot for cerebral rSO2 Variability between Left and Right cerebral rSO2.
agreement between left and right rSO2 readings in terms of variability. The cerebral rSO2 readings in the distal shunt malfunctions had greater variability between left and right rSO2 readings compared with subjects with proximal shunt malfunction. The cerebral rSO2 readings in the proximal

Fig. 5 Bland-Altman plot for repeated measures of cerebral rSO2 readings from 2 subjects.
shunt malfunctions had greater uniformity between the left and right hemispheres. These results lend support to the possible use of variability of left and right cerebral rSO2 measurements in distal vs proximal malfunctioning shunts.
This Bland-Altman plots contrast that performed by Zuluaga et al [22]. In their analysis, they treated repeated measures from the same subject as if from different subjects and ignored the strong correlation among them. We illustrate this point using data from 2 subjects (Fig. 5). It is clear that patterns of agreement are distinct for the 2 subjects. Although there are 491 pairs, they definitely should not be treated as 491 independent pairs.
Association between cerebral rSO2 and patient characteristics
There was no significant difference in readings between subjects with and without developmental delay (left, P = .27; right, P = .68) and between subjects with and without seizures (left, P = .22; right, P = .68). Finally, there did not appear to be any trend in readings across age (left, P = .83; right, P = .87). There was no significant difference in intrasubjects in terms of developmental delay (left, P = .27; right, P = .68), seizures (left, P = .22; right, P = .68), or age (left, P = .83; right, P = .87) (Table 1).
Discussion
The results of this study indicate that left and right cerebral rSO2 readings in subjects with hydrocephalus who
have malfunctioning shunts and altered cerebrovascular autoregulation provide reliable and consistent rSO2 cerebral tissue monitoring.
The intrasubject variation of rSO2 readings was small relative to the intersubject variations. This suggests that the readings were consistent within each patient but that large variations existed between patients. The variation between subjects was also smaller for the right forehead, as can be seen from the small SDs from the overall mean. Large intersubject variations, despite small intrasubject variations, suggest that the rSO2 readings are consistent within each subject.
Baseline left cerebral rSO2 readings were higher than right cerebral rSO2 readings. However, in subjects with left- sided shunts, this was not statistically significant, presum- ably because of a low sample size or large variability. Right cerebral rSO2 readings were also lower than left cerebral rSO2 NIR readings in patients with right-sided shunts. However, regardless of the location of the shunt (right vs left), right cerebral rSO2 readings were always lower, similar to our NIRS study findings in functioning shunts [27]. An explanation for the overall right cerebral rSO2 readings being lower may be the result of greater number of subjects with right-sided shunts (77%) or may represent increased oxygen consumption, extraction, or decreased oxygen supply [26- 29]. The higher left cerebral rSO2 readings can be interpreted as decreased oxygen extraction/consumption or increased oxygen supply [27-29].
The rSO2 intraclass and interclass analyses in this study had greater fluctuation and variation in both areas of analysis. This greater fluctuation in cerebral rSO2 could be the result of altered cerebrovascular autoregulation and cerebrovascular response to the degrees of malfunctioning shunt and ICP [14,19,22,23]. Intracranial pressure changes are dependent on the operational state of the malfunctioning shunt, proximal or distal shunt malfunction, or partial vs complete shunt blockage [14,19,22-25]. This degree of ICP change produces a greater variation in cerebral blood flow, metabolism, and oxygen extraction, producing the greater fluctuation in the rSO2 observations in our study. The distal shunts physiologically would be expected to have a greater variation in cerebral rSO2 readings than the proximal malfunctioning shunt, as demonstrated in Table 1. Numerous studies have demonstrated that proximal malfunctioning shunts have greater ICP pressure because the malfunctioning is at the valve or proximal to the valve, producing a significantly greater increased ICP pressure because of a greater back pressure on a closed system “Monro-Kelly doctrine” that produces less compliance or increased stiffness of the ventricles producing decrease or altered brain perfusion. In the proximal malfunctioning shunt, this greater ICP would be expected to produce a greater decrease in tissue perfusion and oxygenation with increased tissue anaerobic metabolism producing less variation in the cerebral rSO2 readings. In the distal malfunctioning shunts, the blockage is after the valve in the tubing system with
functioning proximal siphoning of the CSF but less back- pressure on the shunt system and the brain. The distal malfunctioning shunt produces a greater degree of ICP fluctuations compared with proximal malfunctioning shunts producing a greater cerebral compliance and perfusion compared with proximal shunt malfunction. This ultimately results in improved cerebral perfusion, oxygenation, and tissue metabolism, as demonstrated by greater variation in cerebral rSO2 readings in distal compared with proximal malfunctioning shunts. The caveat is in a complete proximal or distal malfunctioning shunt; the degree of ICP fluctuation would be expected to be obvious in a distal greater than proximal malfunctioning shunt. In a partial or incomplete distal or proximal malfunctioning shunt, the cerebral rSO2 reading variation would depend on the varying degrees of intracranial cerebral pressure effect on the brain architecture and perfusion.
Low cerebral rSO2 readings or trends reflect an increase in oxygen extraction and debt as a result of increased metabolism, decreased perfusion, and/or stagnant perfusion [25-29]. High rSO2 saturation or trends may be indicative of increased perfusion, decreased tissue bed metabolism, or less oxygen extraction. These baseline cerebral rSO2 values are dependent on many subject-specific factors. Regional oxygen saturation trending is reflective of the subject’s overall clinical state, perfusion, and tissue-specific factors, all integral components for the interpretation of this rSO2 values [23-27]. These cerebral rSO2 readings and trends must be interpreted in relationship to the clinical state of the subject, tissue perfusion, tissue metabolism, and oxygen extraction [24-28].
In the present study, as in prior cerebral rSO2 monitoring in hydrocephalus, we have demonstrated a wide and dramatic variation in the individual and overall subject’s rSO2 readings from the expected cerebral rSO2 readings as demonstrated in normal functioning pediatric brain tissue [22-27]. This is the first study to demonstrate, in most subjects with hydrocephalus, a wide cerebral rSO2 variation between the individual’s cerebral hemispheres. These cerebral rSO2 readings and trends, as in Figs. 2 and 3, must be interpreted in relation to the subject’s developmental state, neurologic function, brain’s architecture, ICP, cerebral tissue perfusion, oxygen demand, and extraction. These high cerebral rSO2 values may represent increased blood flow or, more likely, decreased oxygen extraction and metabolism, especially in the high 90% range [11,12,15,26-29]. The low cerebral rSO2 trending can be interpreted as, possibly, decreased cerebral tissue blood flow, or increased metabolic state, or increased oxygen consumption and extraction [26- 29]. These wide cerebral rSO2 variations demonstrated the varying functional state of the cerebral tissue in response to fluctuating ICP with resultant perfusion alterations in patients with hydrocephalus who have malfunctioning shunts. Cerebral rSO2 readings in this study demonstrated the varying degrees of abnormal tissue perfusion in the malfunctioning shunts, individually and as a group.
The subject’s rise in ICP is multifactorial and dependent on the individual subject’s degree of hydrocephalus, functionality of their shunt, cerebral metabolism, and resting ICP, as well as the patients’ brain development [2,7,14,15,19,26-29]. This subsequent increased ICP and its physiological effects are subject specific with varying effects depending on the degree of decrease cerebral blood flow, regional perfusion, and oxygen delivery [2,14,5,19,24-29]. This secondary effect of declining perfusion may increase the cerebral tissue oxygen extraction and anaerobic metabolism and result in impairment of tissue oxygen saturation. These cumulative factors manifest in unique and patient-specific cerebral rSO2 values, as demonstrated in our study. In Fig. 2, the individual subject’s cerebral rSO2 tissue trending demonstrates the subject’s asymmetrical cerebral dynamics between the left and right hemispheres. Cerebral metabolism between hemispheres in these particular subjects, especially during malfunction periods, is unequal. We have demonstrated the unique cerebral metabolism between subjects and within the subject’s own cerebral hemispheres. This study demonstrates that the use of cerebral rSO2 tissue monitoring as an adjunct tool in the clinical decision tree for malfunctioning shunts cannot be generalized for pediatric patients with hydroceph- alus who have malfunctioning shunt. Future studies in the use of cerebral rSO2 tissue monitoring in subjects with malfunctioning shunt warrant comparing the patient’s cerebral rSO2 tissue readings when the shunt is functioning with those when the shunt is malfunctioning.
Limitations
Convenience patient Selection process in an emergency setting has its own bias. Our study was a preliminary feasibility and analysis for continuous cerebral rSO2 monitoring in the pediatric patients with hydrocephalus who have malfunctioning shunts in the emergency setting. Not monitoring these patients after surgical repair was not the primary intent of this study and not monetarily feasible during the study period.
Conclusion
We demonstrate reliable left and right cerebral rSO2 readings in pediatric patients with malfunctioning shunts. Our study showed the asymmetrical hemispheric cerebral dynamics and the individual uniqueness of cerebral regional rSO2 readings in the pediatric hydrocephalic brain with malfunctioning shunt. These findings confirm the necessity for further studies in cerebral rSO2 monitoring for malfunctioning shunts and warrant intra- subject analysis during periods of functioning and malfunctioning shunts.
References
- Kirkpatrick M, Engleman H, Minns RA. Symptoms and signs of progressive hydrocephalus. Arch Dis Child 1989;64(1):124-8.
- Petrella G, Czosnyka M, Keong N, et al. How does CSF dynamics change after shunting? Acta Neurol Scand 2008;118(3):182-8.
- Browd SR, Ragel BT, Gottfried ON, Kestle JR. Failure of cerebrospinal fluid shunts: part I: obstruction and mechanical failure. Pediatr Neurol 2006;34(2):83-92.
- Zorc JJ, Krugman SD, Ogborn J, Benson J. Radiographic evaluation for suspected cerebrospinal fluid shunt obstruction. Pediatr Emerg Care 2002;18(5):83-92.
- Key CB, Rothrock SG, Falk JK. Cerebrospinal fluid shunt complica- tions: an emergency medicine perspective. Pediatr Emerg Care 1995;11(5):265-73.
- Barnes NP, Jones SJ, Hayward RD, Harkness WJ. Ventriculoper- itoneal shunt block: what are the best predictive clinical indicators? Arch Dis Child 2002;87(3):198-201.
- Malm J, Kristensen B, Fagerlund M, Koskinen LO, Ekstedt J. Cerebrospinal fluid shunt dynamics in patients with idiopathic adult hydrocephalus syndrome. J Neurol Neurosurg Psychiatry 1995;58: 715-23.
- Iskandar BJ, McLaughlin C, Mapstone TB, et al. Pitfalls in the diagnosis of ventricular shunt dysfunction: Radiology reports and ventricular size. Pediatrics 1998;101:1031-6.
- Hall P, Adami HO, Trichopoulos D, Pederson NL, Lagiou P, et al. Effect of low doses of ionising radiation in infancy on Cognitive function in adulthood: Swedish population based cohort study. BMJ 2004;328(7430):19.
- Smyth MD, Narayan P, Tubbs RS, Leonard JR, Park TS, et al. Cumulative diagnostic radiation exposure in children with ventricu- loperitoneal shunts: a review. Childs Nerv Syst 2008;24:493-7.
- Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth 2009;103(Suppl 1):i3-i13.
- Watzman HM, Kurth CD, Montenegro LM, Rome J, Stephen JM, Nicolson SC. Arterial and venous contributions to near-infrared cerebral oximetry. Anesthesiology 2000;93:947-53.
- Petrova A, Mehta R. Near-infrared spectroscopy in the detection of regional tissue oxygenation during hypoxic events in preterm infants undergoing critical care. Pediatr Crit Care Med 2006;7: 449-54.
- Eide PK. Intracranial pressure parameters in idiopathic normal pressure hydrocephalus patients treated with ventriculoperitoneal shunts. Acta Neurochir (Wien) 2006;148:21-9.
- Soul JS, Eichenwald E, Walter G, et al. CSF removal in infantile posthemorrhagic hydrocephalus results in significant improvement in cerebral hemodynamics. Pediatr Res 2004;55:872-6.
- Drayna P, Abramo TJ, Estrada C. Near-infrared spectroscopy in the critical setting. Pediatr Emerg Care 2011;27(5):432-9.
- Gomez H, Torres A, Polanco P, Kim HK, Zenker S, Carlos J, et al. Use of non-invasive NIRS during a vascular occlusion test to assess dynamic tissue O2 saturation response. Intensive Care Med 2008;34(9):1145.
- Murayama H. Measurement of cerebral-oxygenation status when commencing cardiopulmonary bypass in pediatric open-heart surgery. Ann Thorac Cardiovasc Surg 2006;12(2):105-12.
- Weerakkody RA, Czosnyka M, Zweifel C, et al. Slow vasogenic fluctuations of intracranial pressure and cerebral near infrared spectroscopy–an observational study. Acta Neurochir (Wien) 2010;152:1763-9.
- Estrada CM, Abramo TJ. Near-infrared spectroscopy in the pediatric hydrocephalus population. US Pediatrics 2008;4(1):69-70.
- R Development Core Team (2010). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available at: http://www.R- project.org.
- Zuluaga MT, Esch M, Cviianovich N, Gupta N. Diagnosis influences response of cerebral near infrared spectroscopy to Intracranial hypertension in children. Pediatr Crit Care Med 2010;11(4):514-22.
- Wagner BP, Pfenninger J. Dynamic cerebral autoregulatory response to blood pressure rise measured by near-infrared spectroscopy and intracranial pressure. Crit Care Med 2002;30(9):2014-21.
- Ejike JC. cerebral oxygenation in neonatal and pediatric patients during veno-arterial Extracorporeal life support. Pediatr Crit Care Med 2006;7(2):154-8.
- Olsson C, Thelin S. Regional cerebral saturation monitoring with near- infrared spectroscopy during selective antegrade cerebral perfusion: diagnostic performance and relationship to postoperative stroke. J Thorac Cardiovasc Surg 2006;131:371-9.
- Plachky JHS, Volkmann M, Martin E, et al. Regional cerebral oxygen saturation is a sensitive marker of Cerebral hypoperfusion during orthotopic liver transplantation. Anesth Analg 2004;99:344-9.
- Dunham CM, Sosnowski C, Porter JM, Siegal J, Kohli C. Correlation of noninvasive cerebral oximetry with cerebral perfusion in the severe head injured patient: a pilot study. J Trauma 2002;52:40-6.
- Belli A, Sen J, Petzold A, Russo S, Kitchen N, Smith M. Metabolic failure precedes intracranial pressure rises in traumatic brain injury: a microdialysis study. Acta Neurochir (Wien) 2008; 150:461-9.
- Al-Rawi P, Kirkpatrick P. tissue oxygen index: thresholds for cerebral ischemia using near-infrared spectroscopy. Stroke 2006;37: 2720-5.


