Clinical evaluation compared to the pulse indicator continuous cardiac output system in the hemodynamic assessment of critically ill patients
a b s t r a c t
Objective: The objective was to assess the effects of pulse indicator Continuous cardiac output catheterization on the management of critically ill patients and the alteration of therapy in intensive care units.
Methods: One hundred thirty-two patients with primary physiological abnormalities of hypotension or hypoxemia were evaluated. Prior to catheterization, physicians were asked to complete a questionnaire that collected information regarding predictions of the ranges of several Hemodynamic variables and plans for therapy. After catheterization, each chart was reviewed by a panel of intensive care attending physicians to determine the possibility of altering the therapy.
Results: Overall correct classification of the key variables ranged from 46.0% to 65.4%. Catheterization results prompted alterations in therapy for 45.5% of patients. The fellows were less accurate in predicting hemodynamic values for patients whose diagnoses were unknown, and the primary abnormality was hypotension. There was significant difference in the physicians’ abilities to predict the hemodynamics for the subgroups with and without acute myocardial infarction. When the patients were divided into 3 subgroups by Acute Physiology and Chronic Health Evaluation II and Sepsis-related Organ Failure Assessment scores, the fellows had the most difficulty predicting the variables of the moderately ill patients in the middle subgroup, which led to the greatest percentage of therapy alterations for this subgroup; and this difference was significant.
Conclusions: The hemodynamic variables obtained from pulse indicator continuous cardiac output catheteri- zation improved the accuracy of bedside evaluations and led to alterations in therapeutic plans, particularly among the moderately ill patients with hypotension or unknown diagnoses.
(C) 2014
Introduction
The optimal management of cardiopulmonary physiological disorders among critically ill patients requires an accurate assessment of hemodynamic status. The introduction of pulmonary artery catheter- ization (PAC) in the 1970s led to an extreme expansion of the field of hemodynamic monitoring [1,2]. Pulmonary artery catheterization has been proven to always be superior to careful clinical evaluation including physical examination, laboratory examination, and chest roentgenogram in terms of determining hemodynamic status; and it has been shown that the measurements obtained by PAC often prompt changes in therapy [3-9].
However, controversies regarding the overuse of PAC for hemody- namic monitoring exist [10-12]. The effect of PAC on the improvement of patient outcomes is still debated, and the assistance that PAC provides is partially offset by the high associated risk of serious complications and mortality. Recently, a variety of advanced hemodynamic monitoring
? Conflict of interest: The authors have no potential conflicts of interest to disclose.
?? Financial support: This study was not supported by any foundation.
* Corresponding author. Tel./fax: +86 10 64227295.
E-mail address: [email protected] (G. Li).
techniques that are safer and less invasive have been generally used in clinical settings as substitutes for PAC. Among these techniques, the pulse indicator continuous cardiac output (PiCCO) system has been extensively extremely used and provides accurate assessments of constant and dynamic hemodynamic statuses, provides aid in the determination of immediate and subsequent therapies, and correlates well with PAC measurements [13-17].
Despite the widespread use of the PiCCO system, there are few data documenting the superiority of this system in the assessment of hemodynamic status over that of careful clinical evaluation; and the information obtained from PiCCO alters the management of critically ill patients. Our study was designed to prospectively appraise whether PiCCO provides additional useful information beyond that obtained by careful clinical evaluation. Additionally, we evaluated the frequency with which the results of PiCCO resulted in alteration of planned therapies.
Methods
Study site and patients
This prospective evaluation of PiCCO catheterization was per- formed in the intensive care unit (ICU) of the China-Japan Friendship
http://dx.doi.org/10.1016/j.ajem.2014.03.023
Hospital between February 2008 and June 2013. One hundred eighty- three consecutive patients with primary physiological abnormalities of hypotension or hypoxemia were entered into this study. After the
Table 2
Major options Minor options
exclusion of patients with insufficient data, the data from 132 remaining patients were analyzed. Hypotension was defined as a systolic blood pressure (SBP) less than 90 mm Hg or a decrease in SBP of greater than 40 mm Hg compared to baseline. Hypoxemia represented impaired oxygenation and was defined as PaO2/FIO2 less than or equal to 300. Patients meeting the following exclusion criteria were excluded:
(1) younger than 18 years; (2) contraindications to catheterization, including concomitant infection and arterial grafting; (3) history of hemorrhagic shock; (4) moribund state or inability to obtain informed consent; and (5) conditions likely to render the PiCCO measurements inaccurate, including large aortic aneurysms, Intracardiac shunts, and significant mitral/tricuspid regurgitation [18].
Experimental protocols
A 5F thermistor-tipped catheter (Pulsiocath PV2015L20, PiCCO plus; Pulsion Medical Systems, Munich, Germany) was inserted into the femoral artery of the patient. A double-lumen central venous catheter was inserted into the internal jugular vein or subclavian vein. Three central venous injections of 15-mL boluses of cold isotonic saline were injected within 7 seconds, PiCCO was measured at each of these time points, and the values analyzed were the average of these 3 consecutive measure- ments. All operations were performed according to this procedure by ICU physicians under the supervision of fellows, and all catheter positions were confirmed via standard portable chest radiograph.
Data collection
Prior to the insertion of the PiCCO catheter, the team of ICU physicians, which consisted of a critical care attending physician, a critical care fellow, and a resident, was required to complete a questionnaire. The questionnaire collected information from the ICU physician team regarding the purpose of catheterization (diagnostic or monitoring), the primary physiological abnormality of the patient (hypotension or hypoxemia), and the major clinical indication for PiCCO (septic shock, acute respiratory distress syndrome [ARDS], acute myocardial infarction [AMI], congestive heart failure, hypovolemic shock, pulmonary edema, pancreatitis, pulmonary embolism, etc). The physician team was then asked to predict the ranges of the key hemodynamic variables of cardiac index (CI), global end-diastolic index (GEDI), systemic vascular resis- tance index (SVRI), and extravascular lung water index (EVLWI) asked on all previous clinical information (Table 1).
Additionally, the ICU team was also asked to indicate a plan for therapy based on the predicted hemodynamic profile by selecting a plan from the list of potential therapeutic options presented in Table 2.
After catheterization and the first measurement of the hemody- namic variables, a review panel composed of 2 critical care attending physicians reevaluated the hemodynamic data to determine whether alterations to the predicted therapy plan should be made.
Major changes in therapy were defined as changes in the type of therapy (ie, changes in volume management from Fluid expansion to restriction, changes in vasoactive agents from constrictors to dilators, and the initiation of an inotropic drug), and minor changes were defined as differences within a single type of therapy (ie, the usage of diuretic drugs vs Continuous renal replacement therapy for the fluid
CI (L/[min m2]) GEDI (mL/m2) SVRI (dyn.s.cm-5.m2) EVLWI (mL/kg) Low b3 b680 b1200 <=7
Medium 3-5 680-800 1200-2000 8-12
High N 5 N 800 N 2000 >=13
Volume management Fluid expansion crystalloid infusion
Colloid infusion
Fluid restriction Diuretic drugs
Hemodialysis
Continuous renal replacement therapy Vasoactive medication Constrictor Dopamine
Norepinephrine
Dilator sodium nitroprusside Nitroglycerin Urapidil
Nicardipine Diltiazem
Inotropic agent Inotropic drug Cedilanid
Dobutamine Mirinone
restriction or the use of dopamine vs norepinephrine for vessel constriction). The absence of alterations in anticipated therapy was defined by the absence of adjustments in treatment, and simple alterations in Drug dosages (eg, a change in dobutamine dose from 8 to 12 ug/[kg min] or a change fluid infusion from 300 to 400 mL/h) were not interpreted as alterations in therapy.
Statistical analyses
Statistical analyses were performed with SPSS 17.0 software (SPSS Inc, Chicago, IL). The data are presented as the means +- the standard deviations. ?2 tests were used to compare qualitative data, and the Fisher exact probability test was used for small sample sizes as appropriate. t tests were used to compare quantitative data. All P values at or below .05 were considered statistically significant.
Results
Characteristics of the patients at baseline
One hundred thirty-two patients were entered into the study and underwent PiCCO catheterization. Cases of repeated catheterizations were excluded because the prior results may have influenced the later predictions. The sample included 76 men (57.6%) and 56 women (42.4%) with a mean age of 63.4 years (range, 24-89 years). Eighty- three patients had hypotension, and these patients had significantly lower SBPs and higher heart rates, higher PaO2/FIO2 ratios, and higher lactate concentrations than did the remaining 49 patients who had hypoxemia (Table 3).
Complications of catheterization
Serious complications and problems occurring during insertion were uncommon. The incidence of hemorrhage from the femoral arterial puncture site was 6.8% (9/132). These hemorrhages were reduced or stopped by compression and were predominately observed in the patients with coagulation problems. Ventricular (premature ventricular contractions) and Atrial arrhythmias were observed in 5 patients (3.8%) at the time of cold saline injection, and these arrhythmias recovered within 10 seconds without requiring antiarrhythmic therapy.
Overall predictions of hemodynamic variables
There were 396 sets of predictions of CI, GEDI, SVRI, and EVLWI for the 132 patients. The accuracies of the physicians’ predictions of the 4 hemodynamic variables are indicated in Fig. 1. The physicians were
Characteristics of all patients and the hypotension and hypoxemia subgroups of patients
|
All patients |
Patients of hypotension |
Patients of hypoxemia |
|
|
(n = 132) |
(n = 83) |
(n = 49) |
|
|
Age (y) |
63.4 +- 19.7 |
61.8 +- 23.2 |
66.2 +- 18.1 |
|
Sex(male/female) |
76/56 |
52/39 |
24/17 |
|
18.3 +- 5.5 |
18.9 +- 6.4 |
17.2 +- 5.1 |
|
|
SOFA score |
8.7 +- 3.3 |
9.1 +- 3.6 |
8.0 +- 3.2 |
|
SBP (mm Hg)** |
100 +- 27 |
84 +- 25 |
127 +- 19 |
|
Heart rate* |
113 +- 26 |
119 +- 30 |
102 +- 17 |
|
PaO2/FIO2* |
249 +- 48 |
288 +- 53 |
183 +- 22 |
|
Lactate (mmol/L)** |
3.2 +- 1.9 |
4.1 +- 2.5 |
1.8 +- 1.2 |
*P b .05, **P b .01 between the 2 subgroups at baseline.
able to predict EVLWI in 65.4% (259/396) of the cases, which was significantly better than their predictions of the other 3 variables. Despite the lower percentage of correct predictions of GEDI (182/396, 46.0%), this prediction accuracy was not significantly different from those for CI (198/396, 50.0%) or SVRI (210/396, 53.0%). The accuracies of the attending physicians and the fellows in predicting the 4 hemodynamic variables were not different, but both were higher than those of the residents.
Predictions of the fellows and therapy changes
In 60 (45.5%) of the 132 catheterizations, the information obtained by PiCCO resulted in major or minor changes in therapy. The incidence of total therapy changes was analyzed to determine whether it was correlated with the primary physiological abnormality (Fig. 2) or the purpose of the catheterization (Fig. 3). The fellows were less accurate in predicting all variables within the hypotension subgroup, which led to a significantly higher percentage of changes in therapy (53.0% for the hypotension group vs 32.7% for the hypoxemia group, P = .023). Similarly, predictive accuracies for the subgroup that underwent catheterization for diagnostic purposes were much lower than those for the hemodynamic monitoring subgroup, which led to a greater frequency of alterations to the therapeutic plans (58.6% vs 35.1%, respectively, P = 007).
There were multiple clinical indications for PiCCO catheterization; among these indications, the most frequent were septic shock (40.2%) and ARDS (27.3%). No differences in predictive accuracies for CI, SVRI, or EVLWI between the septic shock and ARDS subgroups were observed (Fig. 4); but GEDI was predicted more accurately for the ARDS subgroup by the fellows, and this difference resulted in a halving of the frequency alterations in therapy (30.6% for the ARDS group vs 60.4%for the septic shock group, P = 009).
The prediction accuracy for the AMI patients averaged 80% across all variables (Fig. 5), which was much higher than that for the patients without AMI and led to a reduction in the percentage of therapies that were altered (23.8% vs 49.5%, respectively, P = 034).

Fig. 1. Prediction accuracies for 4 variables. Significant differences between the attending/ fellow and the resident subgroup are indicated as *P b .05, **P b .01.

Fig. 2. Prediction accuracies of the fellows according to primary physiological abnormality (n = 132). Significant differences between the 2 subgroups are indicated as *P b .05,
**P b .01.
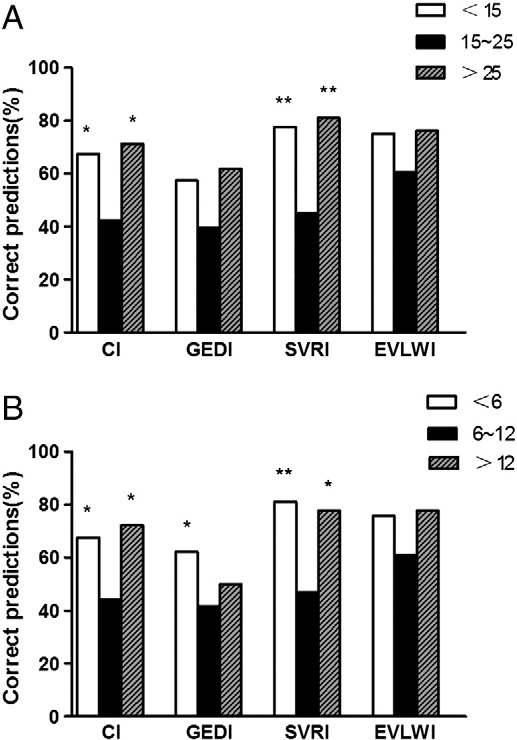
To evaluate whether the accuracies of the predictions were affected by the severity of disease, the patients were divided into 3 subgroups based on Acute physiology and chronic health evaluation II scores (Fig. 6.A). The fellows had the lowest accuracies in predicting CI and SVRI for the group of patients with the midrange APACHE II scores between 15 and 25, and this difference led to percentage of alterations in therapy that was significantly higher than that for all other groups (59.2% vs 30.0%, P = 005; 59.2% vs 28.6%, P = 024). The accuracies the predictions of the fellows for the 4 variables were not different between the high- and low-score groups. We found similar results when the patients were divided into 3 subgroups based on Sepsis-related Organ Failure Assessment (SOFA) scores (Fig. 6.B).
Discussion
Earlier studies have repeatedly shown that PAC is superior to clinical evaluation for the hemodynamic assessment of critically ill patients, and clinical experience and physical examination alone fail to adequately assess as many as half of all significant hemodynamic abnormalities [3-9]. However, PAC is highly invasive and time consuming and is associated with considerable risks of morbidity and mortality [10-12]. Thus, numerous alternative methods of hemodynamic monitoring have been employed. The PiCCO technique has been demonstrated to agree well with the “criterion standard” PAC technique. Pulse indicator continuous cardiac output uses a special arterial thermodilution catheter placed in the femoral artery to measure continuous CO via analysis of the arterial pulse contour [13,14]. The accuracy of PAC has been clinically and experimentally proven [14-17], but few studies of ICU patients have assessed the Therapeutic effect and frequencies of accurate predictions of hemodynamic data under the PiCCO standard.
Our study documented the inability of the physicians who are directly responsible for the care of critically ill patients to accurately predict the hemodynamic variables obtained from the PiCCO system. With the exception of EVLWI, the prediction accuracies for all evaluated variables averaged only approximately 50%. In contrast to

Fig. 3. Prediction accuracies of the fellows according to the purpose of catheterization (n = 132). Significant differences between the 2 subgroups are indicated as *P b .05, **P b .01.

Fig. 4. Prediction accuracies of the fellows for the septic shock and ARDS subgroups (n = 132). Significant differences between the 2 subgroups are indicated as *P b .05, **P b .01.
the results of the study of Connors et al [6], we found that the predictions of the attending physicians and fellows were more accurate than those of the residents. These findings suggest that the physicians’ experience played an important role in the predictions because the residents had less clinical experience and did not optimally use the available clinical information to assess hemody- namic statuses and make clinical judgments.
Our results are similar to those of Forrester et al [19] who reported clinical experience-based prediction accuracies of 81% for CI among 162 AMI patients and 85% for pulmonary capillary wedge pressure among 170 AMI patients. McHugh et al [20] also reported a high correlation between radiographic evidence of left ventricular failure and pulmonary capillary wedge pressure after AMI. Similarly, our study also found a good accuracy of approximately 80% in the prediction of the hemodynamic statuses of AMI patients on the basis of physical examination, laboratory examination, and radiographic findings that limited the value of the PiCCO. Because of the good correlation between the clinical evaluations and the invasive hemodynamic assessments [21], our study suggests that PiCCO catheterization should be indicated only for the small percentage of AMI patients who exhibit diagnostic uncertainty or do not respond to the appropriate initial therapy.
Unfortunately, the fellows were unable to precisely forecast the hemodynamic variables of the patients without AMI, especially when the purpose of the catheterization was diagnostic (the correct prediction rate for all variables averaged 48.3%) or when the patient was hypotensive (the correct prediction rate for all variables averaged 51.4%).
The high frequency of incorrect predictions reported in our study most likely reflected several factors. First, the patients who were hemodynamically monitored were a select group. Only severely critically ill patients whose clinical conditions had deteriorated rapidly and those who did not respond to initial therapy received the PiCCO catheterization. Thus, patients with presumably straight- forward hemodynamic alterations were eliminated from consider- ation. Second, the critically ill patients in the ICU frequently could not express themselves clearly because of abnormal mental status caused by hypotension or hypoxemia; and the background noise in the environment has made accurate characterization of the auscultatory findings extremely difficult. Third, the ICU patients were frequently

Fig. 5. Prediction accuracies of the fellows for the AMI and non-AMI subgroups (n = 132). Significant differences between the 2 subgroups are indicated as *P b .05, **P b .01.
Fig. 6. A, Prediction accuracies of the fellows according to APACHE II scores (n = 132). Significant differences vs the middle subgroup are indicated as *P b .05, **P b .01. B, Prediction accuracies of the fellows according to SOFA scores (n = 132). Significant differences vs the middle subgroup are indicated as *P b .05, **P b .01.
 lying in the supine position with mechanical ventilation, which limited the use of roentgenograms, echocardiograms, and central venous Pressure measurements for the interpretation of interpreting hemodynamic status [8,9]. Fourth, most of the critically ill patients had severe diseases that involved multiple systems that caused ventricular compliance alterations and cardiopulmonary interactions, which made the evaluations of the actual hemodynamic statuses complicated and difficult [5,6]. Fifth, the therapies being provided (eg, vasodilators, diuretics, or catecholamine) at the time of catheteriza- tion might have adversely affected predictive abilities. Finally, the ability to judge clinical situations frequently varies from person to person with different levels of expertise; and this variability affected the assessment of the clinical information to some extent [6,8].
lying in the supine position with mechanical ventilation, which limited the use of roentgenograms, echocardiograms, and central venous Pressure measurements for the interpretation of interpreting hemodynamic status [8,9]. Fourth, most of the critically ill patients had severe diseases that involved multiple systems that caused ventricular compliance alterations and cardiopulmonary interactions, which made the evaluations of the actual hemodynamic statuses complicated and difficult [5,6]. Fifth, the therapies being provided (eg, vasodilators, diuretics, or catecholamine) at the time of catheteriza- tion might have adversely affected predictive abilities. Finally, the ability to judge clinical situations frequently varies from person to person with different levels of expertise; and this variability affected the assessment of the clinical information to some extent [6,8].
Perhaps the most important finding of our study is related to the influences of the PiCCO system on patient management. Total changes in planned therapy occurred in 45.5% of cases, and these alterations in planned therapy were particularly frequent when the diagnosis was not clear or the primary physiological abnormality was hypotension. The risks of hemodynamic monitoring may not be justified when no appropriate therapeutic action is planned on the basis of findings. In the present study, we found that predications were less accurate in hypotension subgroups, especially among the septic shock patients; and this inaccuracy led to a 2-fold increase in the percentage of therapies that were altered (these alterations were predominantly major therapy changes: 28/32) among the ARDS patients (60.4% vs 30.6%, P = 009). Earlier studies have shown lower prediction accuracies and higher percentages of therapies that are changed among patients without AMI [4-6,8,9], particularly among those patients with hypotension; these findings indicate that these patients should be the primary recipients of hemodynamic monitoring.
The ability to predict the hemodynamic statuses of critically ill patients using only clinical parameters might vary with the severity of illness. Celoria et al [3] demonstrated that clinical findings might correlate well with PAC results in cases of single organ system dysfunction, but clinical findings might not correlate well in the
presence of multiorgan system failure. We have observed a similar phenomenon in our studies; that is, the accuracies of predictions are affected by APACHE II/SOFA scores, which might represent the severity of illness, the number of failed organs, and the rate of mortality [22,23]. Using the clinical information, the fellows were able to easily and correctly predict the hemodynamic variables for the patients with illnesses and APACHE II scores less than 15. Similarly, the prediction accuracy was also higher for the extremely severe illness patients with APACHE II scores greater than 25, possibly because of their refractory shock characteristics that included reduced cardiac output and low vascular resistance. Our findings suggest that hemodynamic monitoring via methods such as PiCCO and PAC should be used in a timely manner to identify hemodynamic variables and guide treatment plans for those patients with midrange APACHE II scores who often present as hemodynamically unstable or do not respond to initial treatment.
Limitations
This study did not address the influence of the PiCCO system on outcomes. The factors that influenced outcome were complicated and not necessarily related solely to optimal hemodynamic management. However, we confirmed that the PiCCO technology provided a large amount of hemodynamic information that could not be obtained from clinical evaluations and resulted in alterations in therapy. Additional studies should properly address when and how often such alterations modify outcomes.
Conclusions
This study documented the difficulty of accurately predicting hemodynamics based solely on clinical evaluations. Thus, the information obtained with the PiCCO system improved the accuracies of bedside evaluations and often led to alterations in therapeutic plans, particularly for moderately ill patients with hypotension or unknown diagnoses. We suggest that the PiCCO system was useful and should be indicated for the management of critically ill patients.
Conflict of interest statement
The authors report no conflicts of interest.
The authors thank Pei-Gang Yin, Li-Juan Wu, De-Sheng Chen, Shu-Peng Wang, and Tao Li for excellent clinical assistance.
References
- Sandham JD, Hull RD, Brant RF, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med 2003;348:5-14.
- Harvey S, Young D, Brampton W, et al. Pulmonary artery catheters for adult patients in intensive care. Cochrane Database Syst Rev 2006;19:CD003408.
- Celoria G, Steingrub JS, Maureen VL, et al. Clinical assessment of hemodynamic values in two surgical intensive care units. Arch Surg 1990;125:1036-9.
- Squara P, Bennett D, Perret C. Pulmonary artery catheter: does the problem lie in the users? Chest 2002;121:2009-15.
- Eisenberg PR, Jaffe AS, Schuster DP. Clinical evaluation compared to pulmonary artery catheterization in the hemodynamic assessment of critically ill patients. Crit Care Med 1984;12:549-53.
- Connors AF, Robert MG, Barry AG. Evaluation of right-heart catheterization in the critically ill patient without acute myocardial infarction. N Engl J Med 1983;308:263-7.
- Tuchschmidt J, Sharma OMP. Impact of hemodynamic monitoring in a medical intensive care unit. Crit Care Med 1987;15:840-3.
- Steingrub JS, Celoria G, Maureen VL, et al. Therapeutic impact of pulmonary artery catheterization in a medical/surgical ICU. Chest 1991;99:1451-5.
- Mimoz O, Rauss A, Rekik N, et al. Pulmonary artery catheterization in critically ill patients: a Prospective analysis of Outcome changes associated with catheter- prompted changes in therapy. Crit Care Med 1994;22:573-9.
- Robin ED. The cult of the Swan-Ganz catheter: overuse and abuse of pulmonary
flow catheters. Ann Intern Med 1985;103:445-9.
Wiener RS, Welch HG. Trends in the use of the pulmonary artery catheter in the United States 1993-2004. JAMA 2007;298:423-9.
- Cowie BS. Does the pulmonary artery catheter still have a role in the perioperative period. Anaesth Intensive Care 2011;39:345-55.
- Goedje O, Hoeke K, Michael LA. Continuous cardiac output by femoral arterial thermodilution calibrated pulse contour analysis: comparison with pulmonary arterial thermodilution. Crit Care Med 1999;27:2407-12.
- Oren-Grinberg A. The PiCCO monitor. Int Anesthesiol Clin 2010;48:57-85.
- Monnet X, Persichini R, Ktari M, et al. Precision of the transpulmonary thermodilution measurements. Crit Care 2011;15:R204.
- Hamzaoui O, Monnet X, Richard C, et al. Effects of changes in vascular tone on the agreement between pulse contour and transpulmonary thermodilution cardiac output measurements within an up to 6-hour calibration-free period. Crit Care Med 2008;36:434-40.
- Halvorsen PS, Espinoza A, Lundbhd R, et al. Agreement between PiCCO pulse- contour analysis, pulmonal artery thermodilution and transthoracic thermodilu- tion during off-pump coronary artery by-pass surgery. Acta Anaesthesiol Scand 2006;50:1050-7.
- Giraud R, Siegenthaler N, Park C, et al. Transpulmonary thermodilution curves for detection of shunt. Intensive Care Med 2010;36:1083-6.
- Forrester JS, Diamond GA, Swan HJC. Correlative classification of clinical and hemodynamic function after acute myocardial infarction. Am J Cardiol 1977;39:137-45.
- McHugh TJ, Forrester JS, Adler L, et al. pulmonary vascular congestion in acute myocardial infarction: hemodynamic and radiologic correlations. Ann Intern Med 1972;76:29-33.
- Connors AF, Dawson NV, Shaw PK, et al. Hemodynamic status in critically ill patients with and without acute heart disease. Chest 1990;98:1200-6.
- Knaus WA, Zimmerman LE, Wagner DP, et al. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818-29.
- Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996;22:707-10.
