Diagnostic performance of the BinaxNow Influenza A&B rapid antigen test in ED patients☆
Affiliations
- Department of Emergency Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
Correspondence
- Corresponding author.

Affiliations
- Department of Emergency Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
Correspondence
- Corresponding author.
Affiliations
- Department of Emergency Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
- Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA
Affiliations
- Department of Preventive Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
Affiliations
- Department of Biostatistics, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
Affiliations
- Department of Pathology, Microbiology and Immunology, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
Affiliations
- Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
Affiliations
- Department of Pediatrics, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
- Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
Affiliations
- Influenza Division, Centers for Disease Control and Prevention, Atlanta, GA, USA
Affiliations
- Veterans Affairs, Tennessee Valley Healthcare System, Nashville, TN, USA
- Department of Preventive Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
- Department of Medicine, Vanderbilt University School of Medicine, Nashville, TN 37232-4700, USA
 Article Info
Article Info
To view the full text, please login as a subscribed user or purchase a subscription. Click here to view the full text on ScienceDirect.
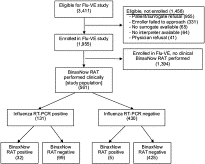
Fig. 1
Flow diagram of study patients.
Fig. 2
Box plot of Ct values for positive RT-PCR influenza tests, comparing Ct values of subjects with a false-negative BinaxNow Influenza A&B RAT and those with a true-positive BinaxNow test. Within each box plot, the center line represents the median, the box contains the interquartile range, and whiskers represent the minimum and maximum values.
Abstract
Objective
The objective of this study is to evaluate the diagnostic performance of the BinaxNow Influenza A&B rapid antigen test (RAT) in emergency department (ED) patients.
Methods
We prospectively enrolled a systematic sample of ED patients older than 6 months with acute respiratory symptoms or nonlocalizing fever during 3 consecutive influenza seasons (2008-2011). Nasal and throat swabs collected by research personnel were tested for influenza by real-time reverse transcription–polymerase chain reaction (RT-PCR). Clinicians independently ordered RATs during clinical care; these specimens were collected by clinical staff and tested for influenza using the BinaxNow RAT. Patients with both a research RT-PCR and clinical RAT were included in the study. Rapid antigen test diagnostic performance was evaluated using RT-PCR as a criterion standard, with preplanned, stratified analysis for subject age, duration of symptoms, influenza subtype, and polymerase chain reaction cycle threshold, which provides a semiquantitative estimate of viral load.
Results
Of 561 subjects enrolled, 131 (23.4%) had a positive RT-PCR, and 37 (6.6%) had a positive RAT. Overall, RAT performance included sensitivity of 24.4% (95% confidence interval [CI], 17.5%-32.9%), specificity of 98.8% (95% CI, 97.1%-99.6%), positive predictive value of 86.5% (95% CI, 70.4%-94.9%), negative predictive value of 81.1% (95% CI, 77.4%-84.3%). Rapid antigen test sensitivities were low for all categories of subject age, symptom duration, influenza subtype, and cycle threshold.
Conclusion
The BinaxNow RAT demonstrated high specificity and poor sensitivity in ED patients selected by treating clinicians for influenza testing. A negative RAT is a poor predictor for the absence of influenza in the ED and should not be used as a criterion to withhold antiviral medications.
To access this article, please choose from the options below
Purchase access to this article
Claim Access
If you are a current subscriber with Society Membership or an Account Number, claim your access now.
Subscribe to this title
Purchase a subscription to gain access to this and all other articles in this journal.
Institutional Access
Visit ScienceDirect to see if you have access via your institution.
☆Support: Supported by CDC Cooperative Agreement U01 IP000184 . Supported by the Office of Academic Affiliations, Department of Veterans Affairs, VA National Quality Scholars Program with resources and the use of facilities at VA Tennessee Valley Healthcare System, Nashville, TN (CD McNaughton).
Related Articles
Searching for related articles..