Clinical effects of adjunctive atropine during ketamine sedation in pediatric emergency patients☆☆☆★★★
Correspondence
- Corresponding author. Tel.: +82 31 787 7575; fax: +82 31 787 4081.

Correspondence
- Corresponding author. Tel.: +82 31 787 7575; fax: +82 31 787 4081.
 Article Info
Article Info
To view the full text, please login as a subscribed user or purchase a subscription. Click here to view the full text on ScienceDirect.
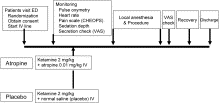
Fig. 1
Study protocol.
Fig. 2
Change in secretion. Between the start and end of the procedure, secretion decreased in the atropine group but increased in the control group (P < .05).
Fig. 3
Change in heart rate. Heart rate was significantly higher in the atropine group compared with the control group (P < .05).
Abstract
Introduction
The prophylactic coadministration of anticholinergics during dissociative sedation has been considered necessary to mitigate ketamine-associated hypersalivation. Given recent conflicting conclusions regarding adjunctive atropine, we compared the incidence of hypersalivation, degree of secretion, and related side effects with atropine or placebo as an adjunct to intravenous (IV) ketamine sedation for children.
Methods
This controlled trial randomized children, 1 to 10 years old, requiring ketamine sedation in a tertiary emergency department to receive 0.01 mg/kg of atropine or placebo, along with IV ketamine (2 mg/kg). A nurse rated preprocedure and postprocedure salivation on a 100-mm visual analog scale and recorded the frequency and nature of airway complications and interventions for hypersalivation.
Results
During 27 months, 140 patients were enrolled. Baseline characteristics did not differ between the 2 groups (P > .05). Secretion was significantly less in the atropine vs placebo group (mean visual analog scale score ± SD, 21.2 ± 13.1 [preprocedure] to 16.5 ± 9.9 [postprocedure] vs 22.4 ± 13.5 [preprocedure] to 27.0 ± 15.9 [postprocedure], respectively; P < .05). Visual analog scale scores greater than 50 were assigned to 7 (9.7%) of 72 and 1 (1.5%) of 68 patients in the placebo and atropine groups, respectively; these patients needed only medical procedures such as suction or airway repositioning. Heart rate was significantly higher in the atropine group compared with the placebo group (P < .05). There were no significant differences between the groups in terms of other adverse events.
Conclusion
Atropine as an adjunct to IV ketamine sedation in children significantly reduced hypersalivation, without providing a clinical benefit.
To access this article, please choose from the options below
Purchase access to this article
Claim Access
If you are a current subscriber with Society Membership or an Account Number, claim your access now.
Subscribe to this title
Purchase a subscription to gain access to this and all other articles in this journal.
Institutional Access
Visit ScienceDirect to see if you have access via your institution.
☆Source of support: This study was supported by grant number 11-2008-037 from the SNUBH Research Fund.
☆☆Trial registration number: NCT00834470.
★Competing interests: The authors report no conflict of interest.
★★Contributor's statement page: Conception: Joong Eui Rhee, Jin Hee Lee, Kyuseok Kim, Tae Yun Kim, and You Hwan Jo. Design: Jin Hee Lee and Yu Chan Kye. Acquisition of data or analysis: Jin Hee Lee, Kyuseok Kim, Tae Yun Kim, You Hwan Jo, and Yu Chan Kye. Interpretation of data: Jin Hee Lee, Yu Chan Kye, and Jin Hee Jeong. Drafting the article or revising: Jin Hee Lee and Yu Chan Kye. Final approval of the version to be published: Jin Hee Lee.
Related Articles
Searching for related articles..


