Initial middle latency auditory evoked potentials index helps to predict resuscitated outcomes in patients with cardiac arrest
a b s t r a c t
Introduction: We speculated that initial middle latency auditory evoked potentials index (MLAEPi) can indicate cerebral function and predict the restoration of spontaneous circulation (ROSC), postresuscitation survival or of neurologic outcomes among patients with cardiac arrest.
Methods: This prospective study included 61 patients with cardiac arrest who received basic life support and did not achieve ROSC until arrival at the emergency center between September 2010 and September 2011. All patients were then administered advanced cardiac life support at the emergency department. Initial MLAEPi was immediately measured using an MLAEP monitor (aepEX plus; Audiomex, Glasgow, Scotland, UK) during the first cycle of advanced cardiac life support. Prediction of the ROSC, survival, and good outcome were investigated.
Results: Sixteen patients achieved ROSC (ROSC group), and 45 did not achieve ROSC at the scene (non-ROSC group). The initial MLAEPi was significantly higher in the ROSC than in the non-ROSC group (33 vs 28, P b
.01). Four survivors in the ROSC group were classified as good outcomes (Cerebral Performance Category 1 and 2). Initial MLAEPi in survivors were significantly higher than that in nonsurvivors (43 vs 29, P b .01). The receiver operating characteristic curves for the initial MLAEPi with area under the curves was 0.75 (95% confidence interval [CI], 0.62-0.88; P b .01) for ROSC, 0.94 (95% CI, 0.88-1.00; P b 0.01) for survival, and 0.96 (95% CI, 0.89-1.03; P b .01) for a good outcome, respectively.
Conclusions: Initial MLAEPi represented by simple numerical values upon presentation at emergency facilities could predict ROSC, survival, and neurologic outcomes among patients with cardiac arrest.
(C) 2013
Introduction
Epochal noninvasive monitoring of patients with cardiac arrest cannot presently predicted cerebral resuscitation during cardiopul- monary resuscitation (CPR). Although the bispectral (BIS) index correlates with neurologic outcomes among patients after postresus- citative therapeutic hypothermia, they cannot predict postresuscita- tion survival or cerebral function during CPR [1-3].
Auditory evoked potentials (AEPs) provide a good indication of consciousness levels in the operative setting during anesthesia [4]. Specifically, cerebral function can be noninvasively monitored by measuring middle latency AEPs (MLAEP), which are derived from AEP
? Competing interests: All authors declare that they have no competing interests. The manuscript, including related data, figures, and tables, has not been published previously and is not under consideration elsewhere.
?? Authors’ contributions: TJ conceived and designed the study. MS provided
technical support, and OS provided final approval of the submitted version of the manuscript.
* Corresponding author. 6-7-1 Nishi-Shinjuku Shinjuku-ku, Tokyo, 160-0023, Japan.
Tel.: +81 3 3342 6111; fax: +81 3 3342 5687.
E-mail address: [email protected] (J. Tsurukiri).
[5]. However, analyzing AEP or MLAEP during emergency care in emergency departments (EDs) using large dedicated instruments is difficult. Data of the AEPs are usually intermittently generated and analyzing waveforms while delivering emergency care, especially Advanced cardiac life support , in an ED might be challenging. The value of AEP monitoring in patients with cardiac arrest is controversial. Although most reports describe studies of small populations during early postresuscitation, AEPs during CPR provide an indicator of restored cerebral blood flow and survival and thus of effective resuscitation [6-9].
The MLAEP monitor (aepEX plus; Audiomex, Glasgow, Scotland, UK) (Fig. 1) is the first mobile MLAEP monitor that can measure the depth of anesthesia [10]. It continuously generates an MLAEP index (MLAEPi), which is a dimensionless number scaled between 100 (wide awake) and 0 (no brain activity), with differences between successive segments of the MLAEP curves constructed from its amplitude. The aepEX is increasingly used as a measurement of both the level of anesthesia and cerebral function instead of BIS values in Intensive care units . We thus speculated that the initial MLAEPi determined upon arrival at EDs can indicate cerebral function during resuscitation, that is, the effectiveness of prehospital
0735-6757/$ – see front matter (C) 2013 http://dx.doi.org/10.1016/j.ajem.2013.02.014

resuscitation, and predict the restoration of spontaneous circulation , postresuscitation survival, or Good neurologic outcomes among patients with cardiac arrest.
Methods
Patients and study design
This study proceeded at the emergency center of Tokyo Medical University Hachioji Medical Center between September 2010 and March 2011 and at Tokyo Medical university hospital between April 2011 and September 2011. The ethics committees at both institutions approved the study design, and written informed consent was obtained from the next of kin of the patients or a posteriori from the patients themselves where possible. All calls concerning cardiac arrest to call centers were immediately transferred to fire department rescue teams located near to the victims. Paramedics immediately started basic life support (BLS) including external cardiac massage, airway management or ventilation, and semiautomatic defibrillation. Japan is divided into distinct medical regions, and victims are dispatched to emergency centers at Regional hospitals. Upon arrival at an emergency center, emergency medical teams comprising paramedics, nurses, residents, and senior physicians specialized in emergency medicine provide ACLS according to current Resuscitation guidelines [11].
We defined cardiac arrest as the absence of both spontaneous breathing and a palpable carotid pulse including asystole, Pulseless electrical activity , or ventricular fibrillation (VF). The present study included all patients with out-of-hospital cardiac arrest who received BLS from bystanders or paramedics within 1 hour of collapse and who did not achieve sequential ROSC until arrival at the emergency center. We excluded patients younger than 15 years; those who arrived more than 1 hour after collapse; those who achieved ROSC before arrival at hospital; and those with trauma, tympanic injury, or terminal diseases.
Intervention
The MLAEPi was continuously calculated using the aepEX plus from information provided by sensor electroencephalogram electrodes affixed to the middle (ground electrode) and right (active electrode) forehead as well as the right mastoid (active electrode)of patients after cleaning the skin with 70% isopropanol. An emergency medical physician also used earphones to determine auditory stimuli.
The AEP was elicited with bilateral click stimuli via earphones at an intensity of less than 60 dB for a nominal frequency of 6.9 Hz. The detected AEPs are consecutively extracted from raw EEG signals that reflect the brainstem AEPs and the MLAEP provided by an internal processor. The aepEX values are closely related to the AEP waveforms and are calculated as the sum of the square roots of the absolute difference between every 2 successive 0.56-millisecond segments of the AEP waveforms. Finally, aepEX continuously generates the MLAEPi, which is a dimensionless number scaled between 100 (wide awake) and 0 (no brain activity).
Patients who did not achieve ROSC until arrival at the emergency center were then administered with ACLS. The procedures of ACLS such as external chest compression, 1 mg adrenaline intravenously, defibrillation, or tracheal intubation were simultaneously started in the ED, and the initial MLAEPi was immediately measured during the first cycle of ACLS. The MLAEPi of patients who did not achieve ROSC in the ED was monitored throughout resuscitation and that of patients who achieved ROSC was monitored until admission to the ICU. None of the patients received sedation or Neuromuscular blockers before the initial MLAEPi was measured in the EDs.
Data collection
The following characteristics of the patients were retrieved from charts and electrocardiograms: age, sex, medical history, body temperature, blood values (pH, lactate concentration, and base excess), first recorded electrical rhythm upon arrival at the emergency center, witnessed cardiac arrest, bystander CPR, resus- citation intervals, and outcomes. A 3-second average of the MLAEPi was entered as the initial MLAEPi. All patients underwent a neurologic examination using the Cerebral performance category score (CPC), before discharge from hospital [12]. Neurologic out- comes dichotomized with a CPC of 1 to 2 or 3 to 5 were considered good and worse, respectively.
Statistical analysis
Data from all eligible patients were analyzed. Continuous variables are shown as median values with interquartile ranges. Between-group differences were statistically assessed using the Mann-Whitney U test for continuous variables and Fisher exact test for categorical variables using Prism version 5.0d (GraphPad Software, San Diego, CA) as appropriate. Categorical variables were calculated as ratios (%) of the frequency of occurrence. Sensitivity, specificity, and positive
survival outcomes“>likelihood ratio at various MLAEPi cut-off points were calculated based on analyses of receiver operating characteristics (ROC) curves, and the maximum Youden index (J = sensitivity + specificity – 1) was determined for each ROC curve. Statistical significance was indicated at the level of 0.05.
Table 2
Comparison of survivors and nonsurvivors in ROSC group
Clinical variables Univariate analysis Survivors
(n = 8)
Nonsurvivors P
(n = 8)
Results
Age (y), median (IQR) 55 (46-74) 77 (62-80) .15
Tympanic temperature (?C), median (IQR)
35.9 (35.3-36.4) 35.1 (34.6-35.3) .04
Clinical characteristics and ROSC
pH, median (IQR) 7.23 (7.19-7.37) 6.86 (6.83-7.06) b.01
We identified 61 patients with cardiac arrest who received BLS
Lactate concentration (mmol/L), 8.0 (6.2-13.4) 12.5 (8.3-19.6) .22 median (IQR)
delivered by paramedics and who did not achieve ROSC until arrival at
Base excess, median (IQR) -6.3 (-7.4
to -4.6)
-17.0 (-20.0
to -10.6)
b.01
the emergency center (Table 1). Continuous MLAEPi monitoring did
Witnessed cardiac arrest, n (%) 7 (88) 6 (75) 1.00
not affect the standard management of ACLS in the EDs. Among the patients, 16 who achieved ROSC (ROSC group) were admitted to our
Resuscitation intervals to start of BLS (min), median (IQR)
4 (3-4) 6 (5-8) .04
ICU, and 45 did not achieve ROSC upon resuscitation in the ED (non- ROSC group). Table 1 compares their baseline characteristics. The initial MLAEPi was significantly higher in the ROSC than in the non- ROSC group.
Survival outcomes
Of 16 patients in the ROSC group, 8 were discharged from hospital (survivors), but the other 8 died (nonsurvivors). Table 2 compares their baseline characteristics. Three survivors in the group with good neurologic outcomes had no disability (CPC 1), and 1 survivor was classified as CPC 2. Two survivors each in the group with worse outcomes were classified as CPC 3 and 4. Eight nonsurvivors were classified as CPC 5. Fig. 2 shows the MLAEPi records of the groups with good and worse neurological outcomes and the non-ROSC group. The initial MLAEPi was significantly higher in the group with a good outcome than in that with a worse outcome (median, 52 vs 31; P b
.01). The initial MLAEPi was also significantly higher in the group with a good outcome than in the non-ROSC group (median, 52 vs 28; P b .01).
Prediction of ROSC, survival, and neurologic outcomes
Fig. 3 shows the ROC curves for the initial MLAEPi to predict ROSC and survival. The areas under the curve (AUCs) were 0.75 (95% confidence interval [CI], 0.62-0.88; P b .01) for ROSC, 0.94 (95% CI, 0.88-1.00; P b .01) for survival and 0.96 (95% CI, 0.89-1.03) for a good outcome. We, therefore, used these values to generate sensitivity and specificity values for the various initial MLAEPi cut-off points shown
MLAEPi, median (IQR) 43 (35-51) 29 (28-30) b.01
in Table 3. Receiver Operator characteristics analysis suggested that cut-offs of an initial MLAEPi of 29, 34, and 38 were the most predictive of ROSC, survival, and good neurologic outcome, respectively. The sensitivity was 100% for initial MLAEPi cut-offs of 24, 33, and 35 to predict ROSC, survival, and good neurologic outcome, respectively.
Discussion
This study is the first to evaluate the MLAEPi of patients with cardiac arrest at emergency centers. Although the major goal of cardiac arrest resuscitation is to improve the rate of postresuscitation survival and neurologic outcomes, effective indicators of cerebral function have not yet been established. Our results showed that the Diagnostic ability of the AUC to predict ROSC is moderate, but the ROC with the greatest AUC indicated a good neurologic outcome: AUC, 0.96 (95% CI, 0.89-1.03). Analyses of ROC curves also suggested that initial MLAEPi cut-offs of 34 or higher and 38 or higher were the most predictive of ROSC and survival, respectively. Moreover, an initial cut- off of MLAEPi 35 or higher had a positive likelihood ratio of 5.0 or higher, and it predicted good survival and neurologic outcome (Table 3). These results indicated that the initial MLAEPi, which detects cerebral function at the ED, would be an indicator of effective resuscitation in the prehospital setting. Moreover, a recent report suggests that the presence of P50 components of the MLAEP, which are considered to be hippocampal CA3 pyramidal cells, might predict good neurologic outcomes after resuscitation [13]. We thus consider that the MLAEPi, which reflects MLAEP morphology, might be a
Clinical characteristics and comparison of patients with or without ROSC
|
Clinical variables |
Total (n = 61) |
Univariate analysis |
|||
|
ROSC (n= 16) |
Non-ROSC (n = 45) |
P |
|||
|
Age (y), median (IQR) |
65 (54-76) |
69 (52-78) |
65 (54-76) |
.96 |
|
|
Male, n (%) |
44 (72) |
12 (75) |
32 (71) |
1.00 |
|
|
Tympanic temperature (?C), median (IQR) |
35.0 (34.5-35.7) |
35.4 (35.0-36.3) |
35.0 (34.4-35.6) |
.09 |
|
|
pH, median (IQR) |
6.94 (6.80-7.12) |
7.11 (6.87-7.23) |
6.91 (6.75-7.10) |
.01 |
|
|
Lactate concentration (mmol/L), median (IQR) |
14.0 (9.9-17.8) |
10.0 (6.6-16.0) |
15.2 (10.8-18.7) |
.02 |
|
|
Base excess, median (IQR) First recorded rhythm on arrival, n (%) Asystole |
-13.6 (-19.9 to -8.6) 35 (57) |
-8.9 (-16.4 to -6.4) 9 (56) |
-15.9 (-21.4 to -10.4) 26 (58) |
.04 1.00 |
|
|
PEA |
23 (38) |
5 (31) |
18 (40) |
.76 |
|
|
VF |
3 (5) |
2 (13) |
1 (2) |
.17 |
|
|
Witnessed cardiac arrest, n (%) |
38 (62) |
13 (81) |
25 (56) |
.07 |
|
|
Bystander CPR, n (%) |
38 (62) |
12 (75) |
26 (58) |
.31 |
|
|
Determined VF during out-of-hospital management, n (%) |
12 (20) |
6 (38) |
6 (13) |
.06 |
|
|
Resuscitation intervals (min), median (IQR) To starting BLS |
7 (3-8) |
5 (3-6) |
7 (4-9) |
.04 |
|
|
To starting ACLS (total, n = 57; ROSC, n = 12; non-ROSC, n = 45) |
35 (29-38) |
31 (23-36) |
35 (29-40) |
.17 |
|
|
MLAEPi, median (IQR) |
29 (25-34) |
33 (29-40) |
28 (23-32) |
b.01 |
|
IQR indicates interquartile range.

Fig. 2. Initial MLAEPi among groups of patients with good and worse outcomes and with good outcomes and non-ROSC group. Boxes plotted groupwise indicate medians and interquartiles with extreme values as whiskers. Good vs worse outcomes (P b .05) and good outcomes vs non-ROSC (P b .05).
reasonable indicator of cerebral function and predict good neurologic outcomes or survival in patients with cardiac arrest.
Our findings suggest that the sensitivity was 100% for initial MLAEPi cut-offs of 24 and 33 to predict ROSC and survival, respectively. None of our patients with cut-offs below these values had ROSC, survival, or good neurologic outcome. Moreover, the likelihood of unFavorable neurologic outcome was 100% when an initial MLAEPi was less than 35 (Table 3). Our emergency medical team must have sufficient knowledge of prognostic factors for postresuscitation survival as well as of changes in the science of resuscitation.
In this study, pH, lactate levels, base excess, and resuscitation intervals to start BLS and MLAEPi were significantly better in the ROSC group than in the non-ROSC group. Moreover, these values excluding lactate concentrations were also significantly better in survivors than in nonsurvivors. Previous reports have confirmed the value of acidosis, Blood lactate levels, base excess, or ammonia for predicting poor prognosis among patients with cardiac arrest [14-17]. Moreover, recent reports described that the values of disseminated intravascular coagulation score, Acute Physiology and Chronic Health Evaluation II, procalcitonin were considered as the independent predictors for poor outcomes [18-20]. The MLAEPi might increase the certainties of bad prognosis leading to stop earlier the resuscitation at the emergency centers in some patients. We found that tympanic temperature was significantly better in survivors than in nonsurvivors, but the median temperature in each group was not low enough to indicate hypothermia. This finding confirms the findings of den Hartog et al
[21] who concluded that spontaneous hypothermia is a predictor of unfavorable neurologic outcomes in patients after resuscitation.
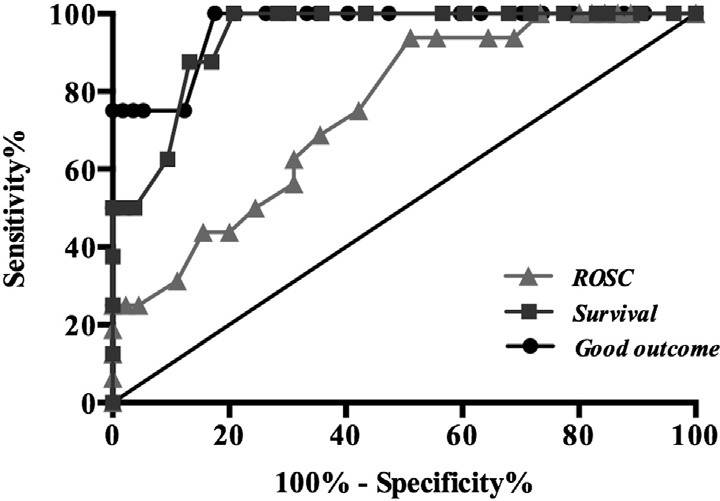
Fig. 3. Optimal ROC curves for initial MLAEPi and ROSC, survival, and good neurologic outcome. Areas under curves are 0.75 (95% CI, 0.62-0.88; P b .01), 0.94 (95% CI, 0.88-
1.00; P b .01), and 0.96 (95% CI, 0.89-1.03) for initial MLAEPi and ROSC, survival, and good neurologic outcomes, respectively. Good neurologic outcome has ROC with greatest AUC.
Table 3
Sensitivity and specificity values at initial MLAEPi cut-off points to predict ROSC, survival, and good neurologic outcome using the respective ROC curve
Initial MLAEPi Sensitivity Specificity Positive likelihood ratio
|
Initial MLAEPi cut-off points to predict ROSC, using the ROSC ROC curve |
|||
|
>=24 |
100.0 |
26.7 |
1.4 |
|
>=29 |
75.0 |
57.8 |
1.8 |
|
>=33 |
50.0 |
75.6 |
2.0 |
|
>=35 |
43.8 |
84.4 |
2.8 |
|
>=37 |
31.3 |
88.9 |
2.8 |
|
>=39 |
25.0 |
95.6 |
5.6 |
|
>=40 |
25.0 |
97.8 |
11.3 |
|
>=44 |
25.0 |
100.0 |
– |
|
Initial MLAEPi >=33 |
cut-off points to predict survival, using 100.0 79.3 |
the survival ROC curve 4.8 |
|
|
>=34 |
87.5 |
83.0 |
5.2 |
|
>=35 |
87.5 |
86.8 |
6.6 |
|
>=37 |
62.5 |
90.6 |
6.6 |
|
>=39 |
50.0 |
96.2 |
13.3 |
|
>=40 |
50.0 |
98.1 |
26.5 |
|
>=44 |
50.0 |
100.0 |
– |
|
Initial MLAEPi cut-off points to predict good outcome, using the good outcome ROC curve |
|||
|
>=35 |
100.0 |
82.5 |
5.7 |
|
>=37 |
75.0 |
87.7 |
6.1 |
|
>=39 |
75.0 |
94.7 |
14.3 |
|
>=40 |
75.0 |
96.5 |
21.4 |
|
>=44 |
75.0 |
98.3 |
42.8 |
|
>=49 |
75.0 |
100.0 |
– |
The MLAEP is derived from AEP, and it reflects the morphology of MLAEP curves. The aepEX plus identifies the brain stem and cortical components, especially positive Pa and negative Nb waves and the MLAEP after auditory stimuli. The MLAEPi is calculated from consistent decreases in amplitude and increases in latency, resulting in individual waves within 144 milliseconds. Analyzing waves in real time during clinical emergency situations is difficult using the MLAEP, as it is usually obtained intermittently. However, the mobile battery- operated aepEX monitor consistently assesses the MLAEP during life- saving procedures while transporting patients within the hospital and for admitted patients. The effectiveness of the MLAEPi in operative and ICU settings has been described. Doi et al [22] found that the MLAEPi was a better indicator of sedation depth than the BIS or any other EEG-based monitoring methods and demonstrated 100% specificity for MLAEPi cut-offs of 37 and 61 for unconsciousness and for being awake during anesthesia, respectively [23]. The value of AEP monitoring in patients with cardiac arrest is controversial, and most published reports have included small case series during early phase of postresuscitation [6-8]. The findings of an animal study suggested monitoring AEPs during CPR could provide an indicator of restored cerebral blood flow and thus effective resuscitation and postresusci- tative survival [9]. Our results confirmed these findings in the clinical setting, although few of our patients had good neurologic outcomes. This study has several limitations, the most notable of which is the small study cohort. Although ventricular fibrillation is a key prognostic factor for patients with cardiac arrest, most of our patients were already in asystole upon arrival at the emergency centers. The appearance of VF rhythms was determined out of hospital in 12 patients, all of whom received Automated external defibrillation. Thus, only patients with recurrent VF arrived at the emergency center with VF rhythms. Secondly, we did not monitor MLAEPi during BLS or ACLS before the patients arrived at hospital. Hayakawa et al [24] recently suggested that ROSC is one of the most important prognostic indicators of a favorable outcome not only for VF but for PEA/systole. Wampler et al [25] also determined that survival rates after hospitalization are higher with than without out-of-hospital ROSC. Resuscitation efforts should thus focus on achieving out-of-hospital
ROSC. Thirdly, we only obtained MLAEPi data during primary resuscitation and had no records from the early phase (within 24 hours) of postresuscitation care. However, the purpose of this study was to assess MLAEPi monitoring for practical prognostication in the setting of an emergency center. We thus limited the study end point to hospital discharge and did not conduct long-term follow-up of the survivors.
Conclusions
The present results indicated that initial MLAEPi represented by simple numerical values upon presentation at emergency facilities could be an indicator of effective prehospital resuscitation and predict ROSC, survival, and good neurologic outcomes among patients with cardiac arrest. Larger studies are essential to further evaluate the ability of MLAEPi monitoring to predict neurologic outcomes in patients with sudden cardiac arrest and to determine reliable cut-offs, especially in the out-of-hospital setting.
Acknowledgments
We sincerely thank Prof Yukio Ikeda of the Department of neurosurgery at Tokyo Medical University Hachioji Medical Center for his assistance.
References
- Shibata S, Imota T, Shigeomi S, et al. Use of the bispectral index during the early postresuscitative phase after out-of-hospital cardiac arrest. J Anesth 2005;19: 243-6.
- Fatovich DM, Jacobs IG, Celenza A, et al. An observational study of bispectral index monitoring for out of hospital cardiac arrest. Resuscitation 2006;69:207-12.
- Chollet-Xemard C, Combes X, Soupizet F, et al. Bispectral index monitoring is useless during cardiac arrest patient’s resuscitation. Resuscitation 2009;80:213-6.
- Jildenstal PK, Hallen JL, Rawal N, et al. Effect of auditory evoked potential-guided anaesthesia on consumption of anaesthetics and early postoperative cognitive dysfunction: a randomized controlled trial. Eur J Anaesthesiol 2011;28:213-9.
- Slabu L, Escera C, Grimm S, et al. Early change detection in humans as revealed by auditory brainstem and middle-latency evoked potentials. Eur J Neurosci 2010;32: 859-65.
- Tiainen M, Kovala TT, Takkunen OS, et al. Somatosensory and brain auditory evoked potentials in cardiac arrest patients treated with hypothermia. Crit Care Med 2005;33:1736-40.
- Sakurai A, Kinoshita K, Moriya T, et al. Reduced effectiveness of hypothermia in patients lacking the wave V in auditory brainstem responses immediately following resuscitation from cardiac arrest. Resuscitation 2006;70:52-8.
- Westeren-Punnonen SM, Ypparila H, Musialowicz T, et al. Recovery of N100 component of auditory event-related potentials and EEG after cardiac arrest during propofol sedation. Br J Anaesth 2005;94:626-9.
- Reid KH, Mullins ER, Iyer VG. Changes in brainstem auditory evoked response latency predict survival after CPR in a rat model of cardiac arrest and resuscitation. Resuscitation 1998;36:65-70.
- Kaul HL, Bhati N. Monitoring depth of anaesthesia. Indian J Anaesth 2002;46: 323-32.
- Nolan JP, Deakin CD, Soar J, et al. European resuscitation council guidelines for resuscitation 2005. Section 4. Adult advanced life support. Resuscitation 2005;67: S39-86.
- Ajam K, Gold LS, Beck SS, et al. Reliability of the cerebral performance category to classify neurological status among survivors of ventricular fibrillation arrest: a cohort study. Scand J Trauma Resusc Emerg Med 2011;19:38.
- Takai N, Oda S, Sadahiro T, et al. Auditory evoked potential P50 as a predictor of neurological outcome in resuscitated cardiac arrest patients. J Clin Neurophysiol 2011;28:302-7.
- Shinozaki K, Oda S, Sadahiro T, et al. blood ammonia and lactate levels on hospital arrival as a predictive biomarker in patients with out-of-hospital cardiac arrest. Resuscitation 2011;82:404-9.
- Lin CH, Chi CH, Wu SY, et al. prognostic values of blood ammonia and partial pressure of ammonia on hospital arrival in out-of-hospital cardiac arrest. Am J Emerg Med 2013;31:8-15.
- Cocchi MN, Miller J, Hunziker S, et al. The association of lactate and vasopressor need for Mortality prediction in survivors of cardiac arrest. Minerva Anesthesiol 2011;77:1-9.
- Takasu A, Sakamoto T, Okada Y. Arterial base excess after CPR: the relationship to CPR duration and the characteristics related to outcome. Resuscitation 2007;73: 394-9.
- Kim J, Kim K, Lee JH, et al. Prognostic implication of initial coagulopathy in out-of- hospital cardiac arrest. Resuscitation 2013;84:48-53.
- Donnino MW, Salciccioli JD, Dejam A, et al. APACHE II scoring to predict outcome in post-cardiac arrest. Resuscitation 2012 [Epub ahead of print].
- Annborn M, Dankiewicz J, Erlinge D, et al. Procalcitonin after cardiac arrest – An indicator of severity of illness, Ischemia-reperfusion injury and outcome. Resuscitation 2013Epub ahead of print.
- den Hartog AW, de Pont AC, Robillard LB, et al. Spontaneous hypothermia on intensive care unit admission is a predictor of unfavorable neurological outcome in patients after resuscitation: an observational cohort study. Crit Care 2010;14: R121.
- Doi M, Morita K, Mantzaridis H, et al. Prediction of responses to various stimuli during sedation: a comparison of three EEG variables. Intensive Care Med 2005;31:41-7.
- Doi M, Gajraj RJ, Mantzaridis H, Kenny GNC. Relationship between calculated Blood concentration of propofol and electrophysiological variables during emergence from anaesthesia: comparison of bispectral index, spectral edge frequency, median frequency and auditory evoked potential index. Br J Anaesth 1997;78:180-4.
- Hayakawa K, Hamasaki T, Sakai T, et al. prognostic indicators and outcome prediction model for patients with return of spontaneous circulation from cardiopulmonary arrest: the Utstein Osaka Project. Resuscitation 2011;82: 874-80.
- Wampler DA, Collett L, Manifold CA, et al. Cardiac arrest is rare without prehospital return of spontaneous circulation. Prehosp Emerg Care 2012;16: 451-5.
