Heart rate variability risk score for prediction of acute cardiac complications in ED patients with chest pain
a b s t r a c t
Background: We aimed to develop a risk score incorporating heart rate variability and traditional vital signs for the prediction of early mortality and complications in patients during the initial presentation to the emergency department (ED) with chest pain.
Methods: We conducted a prospective observational study of patients with a primary complaint of chest pain at the ED of a tertiary hospital. The primary outcome was a composite of mortality, cardiac arrest, ventricular tachycardia, hypotension requiring inotropes or intraaortic balloon pump insertion, intubation or mechanical ventilation, complete heart block, bradycardia requiring pacing, and recurrent ischemia requiring revascularization, all within 72 hours of arrival at ED.
Results: Three hundred nine patients were recruited, and 25 patients met the primary outcome. Backwards stepwise logistic regression was used to derive a scoring model that included heart rate, systolic blood pressure, respiratory rate, and low frequency to high frequency ratio. For predicting complications within 72 hours, the risk score performed with an area under the curve of 0.835 (95% confidence interval [CI], 0.749- 0.920); and a cutoff of 4 and higher in the risk score gave a sensitivity of 0.880 (95% CI, 0.677-0.968), specificity of 0.680 (95% CI, 0.621-0.733), positive predictive value of 0.195, and negative predictive value of 0.985. The risk score performed better than ST elevation/depression and troponin T in predicting complications within 72 hours.
Conclusion: A risk score incorporating heart rate variability and vital signs performed well in predicting mortality and other complications within 72 hours after arrival at ED in patients with chest pain.
(C) 2013
Abbreviations: ACS, acute coronary syndrome; AF, atrial fibrillation; AMI, acute myocardial infarction; AUC, area under the receiver operating characteristic curve; CABG, coronary artery bypass surgery; CI, confidence interval; ECG, electrocardiography; ED, emergency department; HFP, high-frequency power; HRV, heart rate variability; IRB, Institutional Review Board; LFP, low-frequency power; PACS, patient acuity Category Scale; PCI, percutaneous coronary intervention; ROC, receiver operating characteristic; SBP, systolic blood pressure; SGH, Singapore General Hospital; VLFP, very low frequency power; VT, ventricular tachycardia.
? Authors’ contributions: MEHO planned and established the project, including the procedures for data collection; drafted the manuscript; and performed data analysis. KG
drafted the manuscript and performed data collection and data analysis. SF, BH, and KLW performed detailed statistical analysis of the data. KG, ZXK, and NS performed data collection and data analysis. ZL reviewed critical revisions to the manuscript. All authors read and approved the final manuscript.
?? Conflict of interest: The study sponsor had no involvement in the study design, data collection, data analysis and interpretation, and writing of the manuscript. Dr Marcus Ong
and A/Prof Lin Zhiping had a patent filing related to the technology described in the study (Method of predicting acute cardiopulmonary events and survivability of a patient,
Application Number: 13/047,348). Dr Marcus Ong and A/Prof Lin Zhiping also had a licensing agreement with ZOLL Medical Corporation for the technology. All the other authors do not have either commercial or personal associations or any sources of support that might pose a conflict of interest in the subject matter or materials discussed in this manuscript.
? This study was sponsored by ZOLL Medical Corporation.
* Corresponding author. Tel.: +65 63213590; fax: +65 63214873.
E-mail addresses: [email protected] (M.E.H. Ong), [email protected] (K. Goh), [email protected] (S. Fook-Chong), [email protected] (B. Haaland), [email protected] (K.L. Wai), [email protected] (Z.X. Koh), [email protected] (N. Shahidah), [email protected] (Z. Lin).
0735-6757/$ - see front matter (C) 2013 http://dx.doi.org/10.1016/j.ajem.2013.05.005
Patients presenting to the emergency department (ED) with chest pain have a wide spectrum of risk for death and other severe complications, most of which occur in the acute phase after a myocardial infarction [1]. There therefore exists a need for early risk stratification to identify patients who will benefit most from intensive cardiac monitoring, admission to intensive care units, or early revascularization.
Unfortunately, our ability to identify patients at high risk through clinical assessment remains largely inadequate. A number of risk scores have been developed for predicting mortality and/or recurrent infarction and ischemia in acute coronary syndrome (ACS) [2-5]. These risk scores, however, have not been shown to predict complications in the early phase (<=72 hours) of ACS. Furthermore, it remains to be seen if these scores can perform well in ED patients with suspected ACS.
There is an increasing recognition of the relationship between the autonomic nervous system and adverse cardiovascular events [6,7]. Heart rate variability reflects the change in time interval between heartbeats, and HRV from short-term Electrocardiography recordings (5-30 minutes) has been shown to be a strong predictor of adverse cardiovascular events in patients with acute myocardial infarction (AMI) [8,9]. These suggest that HRV measure- ments may serve as a feasible tool for risk stratification of patients with ACS.
Objectives
We aimed to develop a risk score incorporating HRV and traditional vital signs for the prediction of early (<=72 hours) mortality and complications in patients during the initial presentation to the ED with chest pain.
We hypothesize that low HRV is associated with early (<=72 hours) complications in patients presenting to the ED with suspected ACS.
We conducted a prospective observational cohort study. This study was conducted at the Singapore General Hospital (SGH), ED. The SGH ED sees between 300 and 500 patients a day. At SGH ED, all patients are initially triaged by a nurse; and those with airway, breathing, and circulation problems or thought to be possibly unstable and needing Close monitoring are routinely put on ECG monitoring using the LIFEPAK 12 defibrillator/monitor (Physio-Control, Redmond, WA).
Patient recruitment and eligibility
Patients older than 30 years with a primary complaint of nontraumatic chest pain and requiring Continuous ECG monitoring, as assessed by triage, were eligible. We chose the age cutoff as we aim to study patients with a higher likelihood of having ACS. All public hospitals in Singapore use a national Patient Acuity Category Scale (PACS) for triage at the ED. PACS 1 patients are the most critically ill and would therefore be required to be attended to without delay. PACS 2 patients are nonambulant and would appear to be in a stable state on initial cardiovascular examination and are not in danger of imminent collapse. PACS 3 patients are ambulant, and PACS 4 patients are nonemergencies. All patients requiring continuous ECG monitoring, with PACS 1 or 2, were eligible. PACS is a symptom-based triage system and does not have strict physiolog- ical criteria (eg, vital sign cutoff values).
Patients in non-sinus rhythm (asystole, supraventricular arrhyth- mias, ventricular arrhythmias, Complete heart block, and pacemaker rhythm) were excluded. We also excluded cases with a high percentage of artifacts, nonsinus beats, and ectopics combined together (N 30% of recorded tracing). Patients were only recruited during office hours (8:00 AM to 6:00 PM) using convenience sampling. The initial set of vital signs and HRV parameters obtained during triage was recorded for this study. Heart rate variability recordings ranged from 5 to 30 minutes. Ethics approval from Singhealth Centralised Institutional Review Board, Singapore (CIRB ref. no. 2009/871/C) with a waiver of patient consent was obtained. Patients were recruited from November 2006 to December 2007.
Data collection and processing
Electrocardiographic tracings (long lead I, II, and III and 12-lead ECG data) of eligible patients were downloaded daily by a research assistant from the LIFEPAK 12 defibrillator/monitor using the CODE- STAT Suite data review software (version 5.0, Physio-Control). Lead
II ECGs sampled at 125 Hz were extracted as text files for HRV analysis using CODE-STAT and proprietary ECG extraction software (Physio-Control).
The extracted ECG data were preprocessed to reduce the effects of noise and artifacts using a 5- to 28-Hz band-pass filter. This frequency range has been found to enhance the QRS complex against the background noise for easier peak detection [10]. A modified threshold-plus-derivative method was used to detect the QRS complexes, and all ectopics and other nonsinus beats were excluded in accordance with the guidelines outlined by the Taskforce of the European Society of Cardiology [7] using an automatic detection algorithm. Our beat detection and labeling techniques have previously been validated against manually annotated data from the MIT-BIH database [11] and found to perform with high accuracy [12].
Frequency domain HRV variables were obtained in accordance with the guidelines outlined by the Taskforce of the European Society of Cardiology [7]. Frequency domain HRV variables were calculated based on estimates of power spectral density obtained using the Lomb-Scargle periodogram that is commonly used for unevenly sampled sequences. Use of the Lomb-Scargle periodogram eliminates the need for interpolation or resampling of the sequences [13,14]. Power spectral densities within the range of 0.01 to 0.04, 0.04 to 0.15, 0.15 to 0.4, and 0.01 to 0.4 Hz were defined as the very low frequency power (VLFP), low-frequency power (LFP), high-frequency power (HFP), and total power, respectively. The LF/HF ratio was calculated by dividing the normalized LFP (normalized LFP = LFP/ [LFP + HFP] x 100%) over the normalized HFP (normalized HFP = HFP/[LFP + HFP] x 100%) [7].
Vital signs recorded at presentation included heart rates and blood pressures measured using the Propaq CS Vital Signs Monitor (Welch Allyn, Skaneateles, NY) at the ED. Relevant demographics, clinical data and diagnosis, 12-lead ECG data (such as ST elevation/depression), and cardiac enzymes (troponin T) were extracted from the electronic ED Clinical Notes, ECG record, and laboratory records system by 2 inde- pendent research assistants using a standardized data collection form.
Outcomes
The primary outcome was a Composite outcome of mortality, cardiac arrest, sustained ventricular tachycardia (VT), hypotension requiring inotropes or Intraaortic balloon pump insertion, intubation or mechanical ventilation, complete heart block, brady- cardia requiring insertion of a pacing wire, and percutaneous coronary intervention (PCI) or Coronary artery bypass surgery (CABG), all within 72 hours of presentation to the ED. The secondary outcome was complications within 24 hours. Patients were considered to have had a cardiac arrest in the event of developing ventricular fibrillation,
having a sudden unexpected death, or having a resuscitation event requiring cardiopulmonary assistance (chest compressions and/or defibrillation). Sustained VT was defined as VT exceeding 30 seconds or requiring defibrillation. Information regarding the nature of death or any of the above outcomes was extracted from clinical notes. Patients were followed up until discharge or in-hospital mortality.
Statistical analyses
Continuous variables were presented as medians (interquartile range) and were analyzed using the Wilcoxon rank sum test. Categorical variables were presented as numbers (percentage) and analyzed using the ?2 test or the Fisher exact test, respectively, as appropriate.
Vital signs and HRV variables were used in the construction of the
risk prediction score. To facilitate scoring and applicability in the clinical setting, these variables were broken into categories. Vital signs were categorized based on the recognized reference physiological range. Heart rate variability variables were categorized into 3 categories each based on the comparison of data between patients with and without outcomes.
We developed a conceptual model based on our previous work
[15] and existing literature, including age, sex, vital signs, and frequency domain HRV. These factors were first examined by univariate analysis. Afterward, factors with P b .10 by univariate analysis or deemed to be clinically relevant were entered in a backwards stepwise multivariate logistic regression. Variable entry and variable exit cutoffs of P b .15 and P N .20 were used in the logistic
Table 1
Baseline characteristics of patients with and without severe complications within 72 hours of arrival at the ED
Characteristics No complications (n = 284) Complications (n= 25) Age, y
Median (IQR) 62 (52-72) 66 (60-72)
Male sex 197 (69.4) 18 (72.0)
Race
Chinese 182 (64.1) 14 (56.0)
Malay 45 (15.8) 6 (24.0)
Indian 43 (15.1) 4 (16.0)
Others 14 (4.9) 1 (4.0)
Medical history
Ischemic heart disease 149 (52.5) 14 (56.0)
Diabetes 110 (38.7) 12 (48.0)
Hypertension 181 (63.7) 15 (60.0)
Hyperlipidemia 150 (52.8) 12 (48.0)
Prior stroke 34 (12.0) 2 (8.0)
Chronic renal failure 39 (13.7) 5 (20.0)
Congestive heart failure 20 (7.0) 3 (12.0)
Table 2
Number of patients with severe complications within 72 and 24 hours of arrival at the ED
Event <=72 h <=24 h
No. of patients (%)
|
Any severe complication |
25 (8.1) |
21 (6.8) |
|
Mortality |
5 (1.6) |
4 (1.3) |
|
Cardiac arrest or ventricular fibrillation |
7 (2.3) |
6 (1.9) |
|
Sustained VT |
1 (0.3) |
0 (0) |
|
Intubation or mechanical ventilation |
8 (2.6) |
7 (2.3) |
|
Hypotension requiring inotropes or IABP insertion |
7 (2.3) |
7 (2.3) |
|
New complete heart block |
1 (0.3) |
1 (0.3) |
|
Bradycardia requiring pacing |
2 (0.6) |
2 (0.6) |
|
Recurrent ischemia requiring PCI or CABG |
2 (0.6) |
1 (0.3) |
model in order not to miss out any potentially significant predictors. Regression coefficients were recalibrated to adjust for overfitting by multiplying each except the intercept by a factor of (?2 - df)/?2, where df denotes the model degrees of freedom (not including the intercept) and ?2 denotes the test of model ?2 value [16]. After shrinking, coefficients were divided by the smallest coefficient, rounded off to the nearest integer, and assigned as the scoring points for the Risk scoring model. Model calibration was evaluated by the Hosmer-Lemeshow goodness-of-Fit test, and the predictive accuracy of the score (model discrimination) was assessed using the area under the receiver operating characteristic (ROC) curve (AUC) for predicting complications within 72 and 24 hours of arrival at the ED. In addition, differences in the rate of outcomes for increasing risk scores were assessed using the Mantel-Haenszel ?2 test for trend.
The following 3 models were compared to see the AUC: model A- HRV risk score with cutoff of 4 and higher as predictor of acute cardiac complications within 72 hours after presentation, model B-presence of either ST elevation or ST depression as predictor of acute cardiac complications within 72 hours, and model C-troponin T (cutoff, 0.1) as predictor of acute cardiac complications within 72 hours. Predicted probabilities of acute cardiac complications as calculated from the 3 logistics regression models were used to construct ROC curves and corresponding AUC.
Unless otherwise specified, all P values b .05 were considered to indicate statistical significance. Data were stored with Excel (Micro- soft Office 2007; Microsoft, Redmond, WA) and imported into SPSS (version 17.0; SPSS Inc, Chicago, IL), STATA (version 11.1; STATA Corporation, College Station, TX), and R (R Development Core Team [2010]; R: a language and environment for statistical computing; R Foundation for Statistical Computing, Vienna, Austria) software for statistical analysis.
Prior myocardial infarction
79 (27.8) 7 (28.0)
Table 3
Comparison of age, vital signs, and HRV in patients with and without severe
Prior PCI 64 (22.5) 5 (20.0)
complications within 72 hours of arrival at the ED
|
Prior CABG Diagnosis at ED STEMI |
34 (12.0) 43 (15.1) |
2 (8.0) 8 (32.0) |
Variables |
No complications (n = 284) |
Complications (n = 25) |
P value |
|
NSTEMI |
80 (28.2) |
4 (16.0) |
Age, y |
62 (52-72) |
66 (60-72) |
.220 |
|
Unstable angina |
57 (20.1) |
1 (4.0) |
Male sex, n (%) |
197 (69.4) |
18 (72.0) |
.784 |
|
Stable angina |
24 (8.5) |
1 (4.0) |
Vital signs |
|||
|
Unspecified chest pain |
35 (12.3) |
2 (8.0) |
Pulse rate, beat/min |
82 (70-99) |
91 (67-111) |
.534 |
|
23 (8.1) |
4 (16.0) |
Respiratory rate, breath/min |
17 (16-18) |
18 (17-21) |
.037 |
|
|
Other diagnosis+ |
22 (7.7) |
5 (20.0) |
SBP, mm Hg |
139 (117-163) |
112 (81-136) |
b.001 |
|
Disposition from ED |
DBP, mm Hg |
77 (66-91) |
70 (58-85) |
.054 |
||
|
ICU |
54 (19.0) |
11 (44.0) |
HRV variables |
|||
|
HD/ICA |
98 (34.5) |
5 (20.0) |
VLFP, ms2 |
0.118 (0.065-0.180) |
0.090 (0.044-0.164) |
.136 |
|
Ward |
125 (44.0) |
9 (36.0) |
LFP, ms2 |
0.054 (0.031-0.082) |
0.070 (0.027-0.093) |
.786 |
|
7 (2.5) |
0 (0.0) |
HFP, ms2 |
0.054 (0.030-0.099) |
0.068 (0.032-0.174) |
.179 |
Data shown are numbers (percentages) unless otherwise stated.
ICA indicates intensive care area; ICU: intensive care unit; HD: high-dependency ward; NSTEMI: non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
* Gastroesophageal reflux disease, pneumonia, etc.
+ Congestive heart failure, symptomatic anemia, etc.
Data shown are median (25th-75th percentile) unless otherwise stated. DBP indicates diastolic blood pressure; TP; total power.
|
TP, ms2 |
0.268 (0.173-0.369) |
0.291 (0.195-0.361) |
.709 |
|
LFP, n.u. |
46.4 (33.5-64.0) |
36.8 (32.3-44.8) |
.036 |
|
HFP, n.u. |
53.6 (36.0-66.5) |
63.2 (55.2-67.7) |
.036 |
|
LF/HF |
0.87 (0.50-1.78) |
0.58 (0.48-0.81) |
.037 |
Predictors for developing severe complications within 72 hours of arrival at the ED (logistic regression)
|
Variable |
Complications (n = 25) |
No complications (n = 284) |
Unadjusted OR |
P value |
Adjusted OR |
P value |
Transformed |
|
|
No. of patients (%) |
(95% CI) |
(95% CI) |
? coefficient |
|||||
|
Age, y |
||||||||
|
30-59 |
6 (4.7) |
122 (95.3) |
1.00 |
|||||
|
>=60 |
19 (10.5) |
162 (89.5) |
2.39 (0.93-6.15) |
.072 |
||||
|
Sex |
||||||||
|
Male |
18 (8.4) |
197 (91.6) |
1.00 |
|||||
|
Female Heart rate? |
7 (7.4) |
87 (92.5) |
0.88 (0.36-2.19) |
.784 |
||||
|
55-114 |
15 (5.8) |
243 (94.2) |
1.00 |
.008 |
1.00 |
.146 |
||
|
b55 |
4 (19.0) |
17 (81.0) |
3.81 (1.14-12.75) |
.030 |
2.16 (0.50-9.26) |
.300 |
0.77 |
|
|
>=115 |
6 (20.0) |
24 (80.0) |
4.05 (1.438-11.41) |
.008 |
3.12 (0.92-10.63) |
.069 |
1.14 |
|
|
SBP, mm Hg |
||||||||
|
N 140 |
3 (2.2) |
136 (97.8) |
1.00 |
b.001 |
1.00 |
b.001 |
||
|
90-140 |
14 (9.6) |
132 (90.4) |
4.81 (1.35-17.12) |
.015 |
6.54 (1.72-24.84) |
.006 |
1.88 |
|
|
b90 |
8 (33.3) |
16 (66.7) |
22.67 (5.46-94.19) |
b.001 |
24.71 (5.28-115.71) |
b.001 |
3.21 |
|
|
DBP, mm Hg |
||||||||
|
60-120 |
16 (6.2) |
243 (93.8) |
1.00 |
.026 |
||||
|
N 120 |
1 (14.3) |
6 (85.7) |
2.53 (0.29-22.3) |
.403 |
||||
|
b60 |
8 (18.6) |
35 (81.4) |
3.47 (1.38-8.71) |
.008 |
||||
|
Respiratory rate+ |
||||||||
|
0-20 |
19 (6.7) |
264 (93.3) |
1.00 |
1.00 |
||||
|
N 20 VLFP, ms2 |
6 (23.1) |
20 (76.9) |
4.17 (1.50-11.61) |
.006 |
3.70 (1.04-13.15) |
.043 |
1.31 |
|
|
N 0.10 |
10 (5.8) |
161 (94.2) |
1.00 |
.037 |
||||
|
0.04-0.10 |
9 (8.3) |
100 (91.7) |
1.45 (0.57-3.69) |
.437 |
||||
|
b0.04 LFP, ms2 |
6 (20.7) |
23 (79.3) |
4.20 (1.40-12.65) |
.011 |
||||
|
N 0.10 |
4 (8.3) |
44 (91.7) |
1.00 |
.931 |
||||
|
0.05-0.10 |
9 (7.4) |
113 (92.6) |
0.88 (0.26-2.99) |
.833 |
||||
|
b 0.05 HFP, ms2 |
12 (8.6) |
127 (91.4) |
1.04 (0.32-3.39) |
.949 |
||||
|
0.05-0.15 |
5 (4.6) |
103 (95.4) |
1.00 |
.043 |
||||
|
b 0.05 |
11 (7.5) |
135 (92.5) |
1.68 (0.57-4.98) |
.351 |
||||
|
N 0.15 |
9 (16.4) |
46 (83.6) |
4.03 (1.28-12.69) |
.017 |
||||
|
LF/HF ratio |
||||||||
|
N 0.9 |
4 (2.8) |
137 (97.2) |
1.00 |
.015 |
1.0 |
.017 |
||
|
0.3-0.9 |
18 (12.0) |
132 (88.0) |
4.67 (1.54-14.16) |
.006 |
3.78 (1.16-12.31) |
.027 |
1.33 |
|
|
b 0.3 |
3 (16.7) |
15 (83.3) |
6.85 (1.40-33.56) |
.018 |
11.05 (1.92-63.63) |
.007 |
2.40 |
|
+ Breaths per minute.
During the study period, 354 patients were included into the study. Of these, 45 patients were excluded from the study: 22 patients were in non-sinus rhythm (20 patients in atrial fibrillation [AF], 2 patients in VT), 16 patients left against medical advice or were transferred to another hospital for care within 72 hours, and 7 patients were discharged from the ED without admission and were
Risk score? for predicting severe complications within 72 hours of arrival at the ED
|
Characteristics |
Transformed ? coefficients |
Final score |
|
|
Heart rate, beat/min |
|||
|
55-114 |
0 |
0 |
0 |
|
b55 |
0.77 |
1 |
1 |
|
>=115 |
1.14 |
1.48 |
1 |
|
SBP, mm Hg |
|||
|
N 140 |
0 |
0 |
0 |
|
90-140 |
1.88 |
2.44 |
2 |
|
b90 |
3.21 |
4.17 |
4 |
|
Respiratory rate, breath/min |
|||
|
0-20 |
0 |
0 |
0 |
|
N 20 |
1.31 |
1.7 |
2 |
|
LF/HF ratio |
|||
|
N 0.9 |
0 |
0 |
|
|
0.3-0.9 |
1.33 |
1.73 |
2 |
|
2.4 |
3.12 |
3 |
|
lost to follow-up. The baseline characteristics of the remaining 309 patients are shown in Table 1.
The outcomes of patients are shown in Table 2. Table 3 compares patients with and without complications in terms of vital signs and HRV variables. Table 4 lists the variables investigated by univariate analysis. It also shows the unadjusted univariate association and adjusted association from multivariate analysis between the variables and the development of complications within 72 hours of arrival at
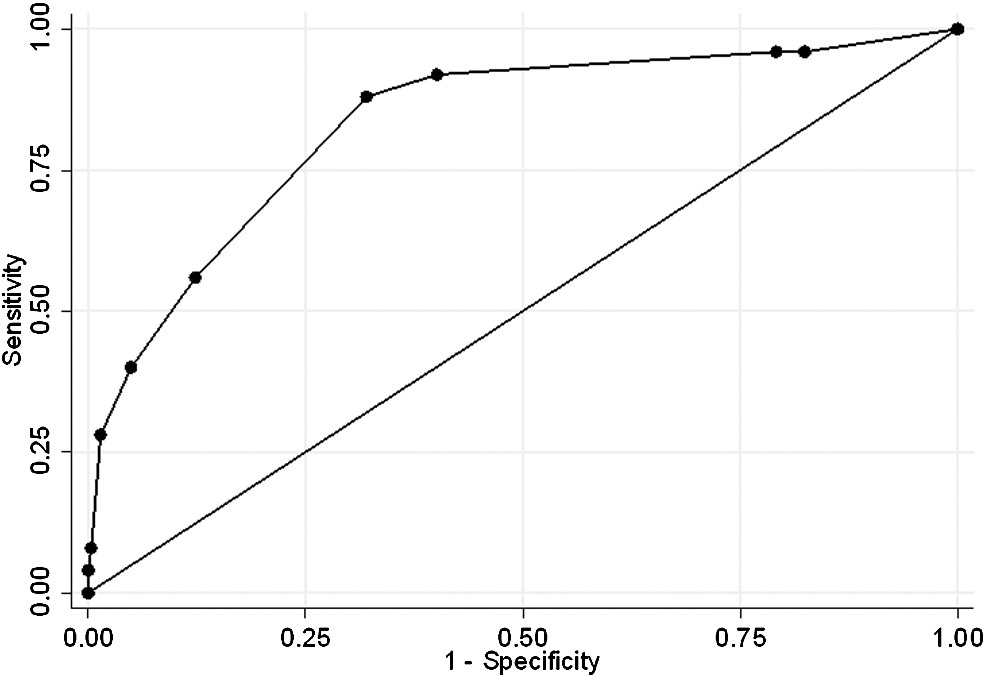
Fig. 1. Receiver operating characteristic analysis of risk score in predicting severe complications with 72 hours at the ED.
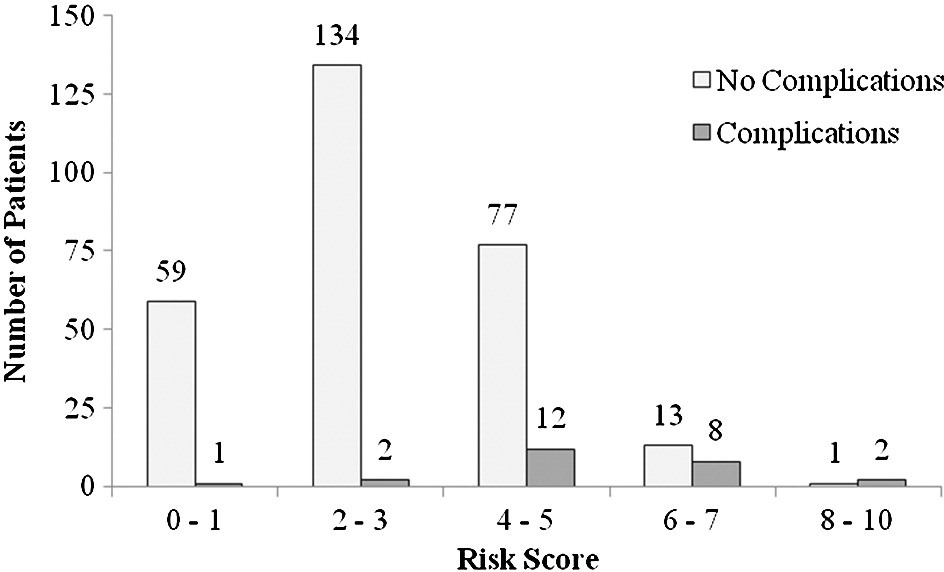
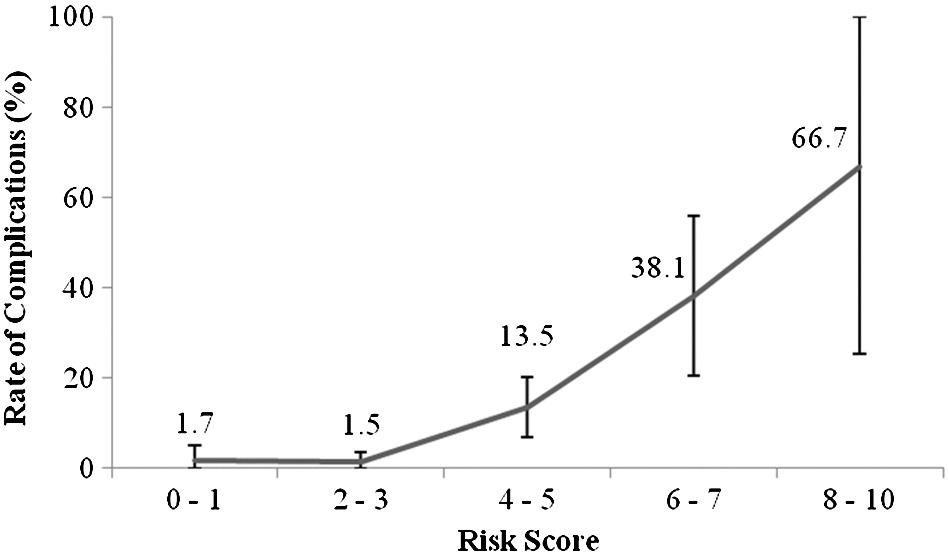
Fig. 2. Distribution of risk score with and without severe complications with 72 hours at the ED.
Fig. 3. Rates of complications within 72 hours in relation to risk score.
the ED. On univariate analysis, abnormal heart rate, lower systolic and diastolic blood pressure, and faster respiratory rates appeared to be associated with the development of early complications, along with lower VLFP, higher HFP, and lower LF/HF ratios. These factors were used in a backward stepwise logistic regression with shrinkage to derive the final model. The risk score points for the final model are shown in Table 5. Of the variables investigated, heart rate, systolic blood pressure (SBP), respiratory rate, and LF/HF ratio were selected for entry in the final risk scoring model. The risk score points for the factors in the final model are shown in Table 5. The Hosmer- Lemeshow goodness-of-fit indicated satisfactory calibration (P =
.432). The risk score ranged from 0 to 10 points (Table 5).
Fig. 1 shows the AUC of the risk score model in predicting complications within 72 hours of arrival at the ED in our derivation data. internal validation demonstrated that the AUC essentially remained unchanged. For predicting complications within 72 hours, the risk score performed with an AUC of 0.835 (95% confidence interval [CI], 0.749-0.920).
Fig. 2 shows the distribution of the risk score and the complication rates within 72 hours in relation to risk scores. There was an increasing rate of complications with increasing risk scores as shown in Fig. 3 (P b .001).
Table 6 gives an overview on the diagnostic statistics performed by models A, B, and C. Model A shows the highest sensitivity of 0.880 (95% CI, 0.677-0.968) and specificity of 0.680 (95% CI, 0.621-
0.733). Model A also performs better with a positive predictive value of 0.195 and a negative predictive value of 0.985 among the 3 models (P b .05)
In this observational cohort study of ED patients with chest pain, patients who developed severe complications within 72 hours of arrival at the ED were found to have lower LF/HF ratios. A risk score incorporating LF/HF ratio, heart rate, SBP, and respiratory rate performed better than ST elevation/depression and troponin T in predicting severe complications within 72 and 24 hours of arrival at the ED. The long-term aim is to develop an objective tool applicable to the population of patients with chest pain that can be used for rapid triage and prognostication during the initial assessment, even before routine blood tests or other more sophisticated results are available. The results of our study are consistent with previous studies that have shown that frequency domain HRV parameters are strongly associated with cardiac mortality and adverse events [17]. Carpeg- giani et al [18] also found that lower HRV was associated with in-
hospital complications in patients with AMI.
It is believed that depressed or low HRV may therefore reflect a decrease in vagal activity directed to the heart and a prevalence of sympathetic mechanisms [7,19], which lead to cardiac instability and
an increased risk for adverse cardiac outcomes. The LF/HF ratio has thus been proposed to be an accurate measure of the shifts in sympathovagal balance, with low LF/HF ratios indicating depressed physiological modulation of the autonomic nervous system [20]. Interestingly, Lombardi et al [21] found significantly increased LF/HF ratios in the early hours after AMI, suggesting a possible overactivation of sympathetic tone with reduced parasympathetic modulation. The finding of low LF/HF ratios in patients who developed early complications in our study may reflect an impaired autonomic response to myocardial ischemia, which predisposes to complications like cardiogenic shock.
In this study, Abnormal vital signs like SBP, respiratory rate, and heart rate were also found to be associated with early complications. This is not an unexpected finding because the derivation studies of various ACS risk score models [2,5,22] report an association between lower SBPs and faster heart rates with short- and long-term mortality in ACS.
Several risk scores for ACS have been developed and validated, including the Thrombolysis in Myocardial Infarction risk score for unstable angina and non-ST-elevation myocardial infarction [3] and the more recently developed global registry of acute coronary events risk score for predicting mortality in-hospital [5] as well as at 6 months [4] in patients with ACS. These scores, however, have not been validated to predict early (<=72 hours) complications in patients with chest pain. The ability to accurately predict which patients will develop such outcomes in the next few days will be ideal for optimal management at the ED, especially in the allocation of limited re- sources for continuous cardiac monitoring. In addition, patients with a high risk score may require more aggressive management strategies [23-25]. A high risk score may therefore serve as an adjunct tool for the selection of patients for early revascularization. The nature of the calculation of the risk score also holds some advantages over existing ACS risk scores. Firstly, the score consists of variables that may be objectively and noninvasively measured without a need for accurate medical and Drug history, which is often not available at the ED setting. The integer point score in our model also allows for simple calculation and ease of use and may be incorporated in devices capable of real time-risk scoring and monitoring.
- Limitations
Several limitations of our study should be acknowledged. Because of the inherent nature of HRV in measuring variations in heart beats, patients in non-sinus rhythm were excluded from this study. Patients in AF, however, only represent a minority of patients, estimated to be approximately 2% to 4% in patients between the ages of 60 and 79 years
[26] and less than 1% in patients younger than 55 years [26]. In this study, 20 (5.6%) of 354 patients were excluded because of AF rhythm.
Not all patients in our study were followed up for a minimum 72 hours. Instead, all patients were followed up until discharge or in-
Discriminatory values of models A, B, and C
|
Diagnostic statistics |
Model A |
Model B |
Model C |
Difference (A - B)? (95% CI for difference) |
Difference (A - C)+ (95% CI for difference) |
P value (A vs B)? |
P value (A vs C)? |
|
Sensitivity |
0.880 |
0.560 |
0.680 |
0.320 (-0.010 to 0.508) |
0.20 (-0.044 to 0.278) |
- |
- |
|
Specificity |
0.680 |
0.606 |
0.504 |
0.074 (-0.006 to 0.154) |
0.176 (0.098 to 0.254) |
- |
- |
|
Positive predictive value |
0.195 |
0.111 |
0.108 |
- |
- |
.011 |
.001 |
|
Negative predictive value |
0.985 |
0.940 |
0.947 |
- |
- |
.025 |
.028 |
Model A: HRV risk score with cutoff of 4 and higher as predictor of acute cardiac complications within 72 hours after presentation. Model B: presence of either ST elevation or ST depression as predictor of acute cardiac complications within 72 hours.
Model C: troponin T (cutoff 0.01) as predictor of acute cardiac complications within 72 hours.
- 95% CI for the difference in diagnostic statistics of model A and model B.
+ 95% CI for the difference in diagnostic statistics of model A and model C.
? For comparison of positive and negative predictive values between any 2 models, only P values are presented, as construction of 95% CI for the difference is not well established.
hospital mortality. Excluding patients who developed complications, a total of 81 (26.2%) of 309 patients were discharged from hospital before 72 hours. None of these patients were discharged against medical advice and they were deemed to be safe for discharge to receive out-of-hospital care. There were no missing ED data.
The number of events in this study was relatively small, with 25 (8.1%) of 309 patients developing complications. This may affect the power of our study when attempting multivariate analyses for several variables for modeling of a risk score. Validation studies will be needed to establish the predictive accuracy of this risk score.
We also did not include other clinical data like ECG changes (eg, ST elevation) and cardiac enzymes (eg, troponins) into the prediction model. This is because we had in mind to incorporate the risk score into a rapid field triage device that could be used in EDs or ambulances. In such a setting, the risk score would be generated using real-time HRV measurements and immediately available vital signs. However, 12-lead ECG and cardiac enzymes would not be so readily available in such a setting.
Reduced LF/HF ratio, lower SBP, and higher respiratory rates were associated with early (<=72 hours) complications in ED patients with chest pain. A risk score incorporating heart rate, blood pressure, respiratory rate, and LF/HF ratio performed better than ST elevation/ depression and troponin T in predicting complications within 72 hours of arrival at the ED. Validation studies will be needed to establish the predictive capabilities of this risk score. There is potential for developing a chest pain triage tool based on HRV.
We would like to thank and acknowledge the contributions from all the physicians and nurses from the Department of Emergency Medicine, Singapore General Hospital.
- Huikuri HV, Castellanos A, Myerburg RJ. Sudden death due to cardiac arrhythmias. N Engl J Med 2001;345(20):1473-82.
- Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, et al. Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation 2000;101(22):2557-67.
- Antman EM, Cohen M, Bernink PJ, McCabe CH, Horacek T, Papuchis G, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA 2000;284(7):835-42.
- Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, et al. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA 2004; 291(22):2727-33.
- Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med 2003;163(19):2345-53.
- Huikuri HV, Makikallio T, Airaksinen KE, Mitrani R, Castellanos A, Myerburg RJ. Measurement of heart rate variability: a clinical tool or a research toy? J Am Coll Cardiol 1999;34(7):1878-83.
- Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17(3): 354-81.
- Fei L, Copie X, Malik M, Camm AJ. Short- and long-term assessment of heart rate variability for risk stratification after acute myocardial infarction. Am J Cardiol 1996;77(9):681-4.
- Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation 1993;88(3):927-34.
- Kohler BU, Hennig C, Orglmeister R. The principles of software QRS detection. IEEE Eng Med Biol Mag 2002;21(1):42-57.
- Moody G: MIT-BIH arrhythmia database directory. http://www.physionet.org/ physiobank/database/mitdb/.
- Padmanabhan P, Lin Z, Ong M, Ser W, Huang G-B. Automatic extraction of HRV sequences from noisy ECG data for reliable analysis and telediagnosis. Paper presented at: Proc 3rd IASTED Int Conf Telehealth; 2007.
- Thong T, Yung I, DP P, Ellingson R, McNames J, Aboy M, et al. Heart rate variability analysis of effect of nicotine using periodograms. Conf Proc IEEE Eng Med Biol Soc 2004;1:294-7.
- Van Dongen H, Olofsen E, VanHartevelt J, Kruyt E. Searching for biological rhythms: peak detection in the periodogram of unequally spaced data. J Biol Rhythms 1999;14(6):617-20.
- Ong ME, Padmanabhan P, Chan YH, Lin Z, Overton J, Ward KR, et al. An observational, prospective study exploring the use of heart rate variability as a predictor of clinical outcomes in pre-hospital Ambulance patients. Resuscitation 2008;78(3):289-97.
- Harrell Jr FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15(4):361-87.
- Singh N, Mironov D, Armstrong PW, Ross AM, Langer A. Heart rate variabil- ity assessment early after acute myocardial infarction. Pathophysiological and prognostic correlates. GUSTO ECG Substudy Investigators. Global Utiliza- tion of Streptokinase and TPA for Occluded Arteries. Circulation 1996;93(7): 1388-95.
- Carpeggiani C, L’Abbate A, Landi P, Michelassi C, Raciti M, Macerata A, et al. Early assessment of heart rate variability is predictive of in-hospital death and major complications after acute myocardial infarction. Int J Cardiol 2004;96(3): 361-8.
- Bigger Jr JT, La Rovere MT, Steinman RC, Fleiss JL, Rottman JN, Rolnitzky LM, et al. Comparison of baroreflex sensitivity and heart period variability after myocardial infarction. J Am Coll Cardiol 1989;14(6):1511-8.
- Malik M, Camm AJ. Components of heart rate variability-what they really mean and what we really measure. Am J Cardiol 1993;72(11):821-2.
- Lombardi F, Sandrone G, Spinnler MT, Torzillo D, Lavezzaro GC, Brusca A, et al. Heart rate variability in the early hours of an acute myocardial infarction. Am J Cardiol 1996;77(12):1037-44.
- Morrow DA, Antman EM, Giugliano RP, Cairns R, Charlesworth A, Murphy SA, et al. A simple risk index for rapid initial triage of patients with ST- elevation myocardial infarction: an InTIME II substudy. Lancet 2001;358(9293): 1571-5.
- Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007;28(13):1598-660.
- Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey Jr DE, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of thoracic surgeons endorsed by the
American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007;50(7): e1-157.
- Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the
management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2008;51(2):210-47.
