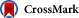ED disposition of the Glasgow Coma Scale 13 to 15 traumatic brain injury patient: analysis of the Transforming Research and Clinical Knowledge in TBI study☆☆☆
Affiliations
- Emergency Medicine and Neurocritical Care, University of Cincinnati, 231 Albert Sabin Way, PO Box 670769, Cincinnati, OH 45267-0769
Correspondence
- Corresponding authors.

Affiliations
- Emergency Medicine and Neurocritical Care, University of Cincinnati, 231 Albert Sabin Way, PO Box 670769, Cincinnati, OH 45267-0769
Correspondence
- Corresponding authors.
Affiliations
- Emergency Medicine and Neurosurgery, University of Cincinnati, 231 Albert Sabin Way, PO Box 670769, Cincinnati, OH 45267-0769
Affiliations
- Emergency Medicine, University of Cincinnati, 231 Albert Sabin Way, PO Box 670769, Cincinnati, OH 45267-0769
Affiliations
- Emergency Medicine, University of Cincinnati, 231 Albert Sabin Way, PO Box 670769, Cincinnati, OH 45267-0769
Affiliations
- Emergency Medicine, University of Cincinnati, 231 Albert Sabin Way, PO Box 670769, Cincinnati, OH 45267-0769
Affiliations
- Emergency Medicine, University of Cincinnati, 231 Albert Sabin Way, PO Box 670769, Cincinnati, OH 45267-0769
Affiliations
- Neurological Surgery, University of California, San Francisco, 1001 Potrero Ave, Building 1 Room 101, San Francisco, CA 94110
Affiliations
- Emergency Medicine, University of California, Davis, 4150 V St, Suite 2100, Sacramento, CA 95817
Affiliations
- Rehabilitation Medicine, Mount Sinai School of Medicine, 1425 Madison Ave, Box 1240, New York, NY 10029
Affiliations
- Seton Brain and Spine Institute, 1400 North IH 35, Suite 300, Austin, TX
Affiliations
- Neurological Surgery, University of Pittsburgh Medical Center, 200 Lothrop St Suite B-400, Pittsburgh, PA 15213
Affiliations
- Erasmus MC, PO Box 2040, 3000 CA, Rotterdam, The Netherlands
Affiliations
- Neurosurgery, Antwerp University Hospital, University of Antwerp, Wilrijkstraat, Edegem, Belgium 102650
Affiliations
- Emergency Medicine, University of California, Davis, 4150 V St, Suite 2100, Sacramento, CA 95817
Correspondence
- Corresponding authors.

Affiliations
- Emergency Medicine, University of California, Davis, 4150 V St, Suite 2100, Sacramento, CA 95817
Correspondence
- Corresponding authors.
 Article Info
Article Info
To view the full text, please login as a subscribed user or purchase a subscription. Click here to view the full text on ScienceDirect.
Fig. 1
Univariable multinomial logistic regression results by ED disposition.
 P < .05; $International normalized ratio; +Odds per year of education.
P < .05; $International normalized ratio; +Odds per year of education.
Fig. 2
Multivariable multinomial logistic regression results by ED disposition.
 P < .05.
P < .05.
Abstract
Objective
Mild traumatic brain injury (mTBI) patients are frequently admitted to high levels of care despite limited evidence suggesting benefit. Such decisions may contribute to the significant cost of caring for mTBI patients. Understanding the factors that drive disposition decision making and how disposition is associated with outcomes is necessary for developing an evidence-base supporting disposition decisions. We evaluated factors associated with emergency department triage of mTBI patients to 1 of 3 levels of care: home, inpatient floor, or intensive care unit (ICU).
Methods
This multicenter, prospective, cohort study included patients with isolated head trauma, a cranial computed tomography as part of routine care, and a Glasgow Coma Scale (GCS) score of 13 to 15. Data analysis was performed using multinomial logistic regression.
Results
Of the 304 patients included, 167 (55%) were discharged home, 76 (25%) were admitted to the inpatient floor, and 61 (20%) were admitted to the ICU. In the multivariable model, admission to the ICU, compared with floor admission, varied by study site, odds ratio (OR) 0.18 (95% confidence interval [CI], 0.06-0.57); antiplatelet/anticoagulation therapy, OR 7.46 (95% CI, 1.79-31.13); skull fracture, OR 7.60 (95% CI, 2.44-23.73); and lower GCS, OR 2.36 (95% CI, 1.05-5.30). No difference in outcome was observed between the 3 levels of care.
Conclusion
Clinical characteristics and local practice patterns contribute to mTBI disposition decisions. Level of care was not associated with outcomes. Intracranial hemorrhage, GCS 13 to 14, skull fracture, and current antiplatelet/anticoagulant therapy influenced disposition decisions.
To access this article, please choose from the options below
Purchase access to this article
Claim Access
If you are a current subscriber with Society Membership or an Account Number, claim your access now.
Subscribe to this title
Purchase a subscription to gain access to this and all other articles in this journal.
Institutional Access
Visit ScienceDirect to see if you have access via your institution.
☆This work was supported by: National Institutes of Health (Bethesda, MD)/National Institute of Neurological Disorders and Stroke: RC2NS069409 —“Transforming Research and Clinical Knowledge in TBI.” National Institutes of Health/National Institute of Neurological Disorders and Stroke: 5T32NS047996 —“Cerebrovascular Fellowship Training Program.”
☆☆This manuscript was an oral presentation at Society for Academic Emergency Medicine: Oral presentation on May 15, 2013 in Atlanta, GA.
Related Articles
Searching for related articles..