SARS-CoV-2 infection and its association with thrombosis and ischemic stroke: a review
a b s t r a c t
This review of current literature provides background to the COVID-19 pandemic, as well as an examination of potential Pathophysiologic mechanisms behind development of thrombosis and ischemic stroke related to COVID-19. SARS-CoV-2 infection is well-documented to cause severe pneumonia, however, thrombosis and thrombotic complications, such as ischemic stroke, have also been documented in a variety of patient demo- graphics. SARS-CoV-2 infection is known to cause a significant inflammatory response, as well as invasion of vas- cular Endothelial cells, resulting in endothelial dysfunction. These factors, coupled with imbalance of ACE2 and RAS axis interactions, have been shown to create a prothrombotic environment, favoring Thromboembolic events. Ischemic stroke is a severe complication of COVID-19 and may be a presenting symptom in some patients.
(C) 2020
Coronaviruses are positive-sense single stranded RNA viruses of the coronaviridae family [1]. The genome of coronaviruses contains open reading frames for 16 non-structural proteins as well as for spike (S), envelope, membrane, and nucleocapsid structural proteins [1]. Four genera exist within the coronaviridae family based on phy- logeny: alpha, beta, gamma, and deltacoronaviruses [1]. Only mem- bers of the alpha and betacoronavirus genera are known to infect humans and can cause clinical presentations ranging from the com- mon cold to severe acute respiratory syndrome (SARS) [1]. Viruses of particular note from the betacoronavirus genus are the SARS- coronavirus (SARS-CoV) and Middle East respiratory syndrome coro- navirus (MERS-CoV) [1]. SARS-CoV is the etiological agent of the 2002 to 2003 SARS epidemic beginning with zoonotic origin in South- ern China and leading to over 8000 confirmed cases with an estimated 9-11% fatality rate [1,2]. MERS-CoV is currently endemic to the Ara- bian Peninsula and has proven to be a dangerous virus of zoonotic or- igin with an estimated 36% fatality rate [1].
December 2019 marked the discovery of a new coronavirus in Wuhan, China after an outbreak of severe pneumonia of unknown ori- gin [3]. Isolation and sequencing of this virus from human airway epi- thelial cells allowed the characterization of the betacoronavirus
Non-standard Abbreviations and Acronyms: ACE2, Angiotensin converting enzyme 2; ANGII, Angiotensin II; ANG1-7, Angiotensin-(1-7); SARS-CoV-2, SARS coronavirus 2; COVID-19, Coronavirus disease 2019; R0, Basic reproductive number; RAS, renin- angiotensin system; S-protein, Spike protein.
E-mail address: [email protected].
named SARS coronavirus 2 (SARS-CoV-2) that is the etiologic agent of coronavirus disease 2019 (COVID-19) [3,4]. Further characterization of SARS-CoV-2 has demonstrated close genomic similarity to several types of bat coronavirus, indicating bats as a likely reservoir for this virus of zoonotic origin [4]. SARS-CoV-2 shares approximately 79% se- quence identity with SARS-CoV, though SARS-CoV-2 has demonstrated a higher rate of transmission than SARS-CoV [4]. Basic reproductive number (R0) is used to represent the transmissibility of a disease and is defined the average number of new cases caused by a single infective person in an unexposed population [5]. The R0 of SARS-CoV-2 and SARS- CoV are estimated at 2.9 and 1.85 for each virus, respectively [5,6]. This is likely due to the presence of asymptomatic or mildly symptomatic transmission of SARS-CoV-2, and its current prevalence in the human population supports the infective potential of this novel coronavirus [5,6]. Since its December 2019 emergence, SARS-CoV-2 has been de- clared a global pandemic by the World Health Organization, and the Centers for Disease Control and Prevention reports over 3 million infec- tions resulting in over 130,000 deaths in the United States alone as of 10 July 2020 [7,8].
-
- Angiotensin Converting Enzyme 2 (ACE2) as the functional receptor of SARS-CoV-2
Viral entry into cells by coronaviruses is mediated through the inter- action between a cell-surface receptor protein and the viral S-protein [9]. Cellular tropism of coronaviruses is dependent on the S-protein- receptor interaction [9] and understanding the tropism of SARS-CoV-2 is the beginning to elucidating the myriad effects this virus may have on human physiology.
https://doi.org/10.1016/j.ajem.2020.09.072
0735-6757/(C) 2020
SARS-CoV-2 shares 73% to 76% amino acid sequence identity in the receptor binding domain of its S-protein with SARS-CoV [4,10], and the amino acid sequence directly interacting with the cell receptor is highly conserved between the viruses [4,10]. SARS-CoV has been previ- ously determined to use the human transmembrane protein angioten- sin converting enzyme 2 (ACE2) as its receptor for viral entry, and SARS-CoV-2 had been speculated to use ACE2 for viral entry as well [9,10]. Several studies have confirmed that ACE2 is the functional recep- tor for SARS-CoV-2 [11-13].
-
- ACE2: its location and role in the renin-angiotensin system (RAS)
Human ACE2 is a transmembrane zinc metalloprotease that acts as a carboxypeptidase in the metabolic degradation of angiotensin I and angiotensin II (ANGII) [14]. ACE2 mRNA is expressed in most tissues of the body, with highest expression in the GI tract, kidney, testes, heart, and lungs [15]. ACE2 protein is found expressed on the surface of lung alveolar epithelial cells, enterocytes of the Small intestine, arte- rial smooth muscle cells, and both arterial and venous endothelial cells, including intracranial vessels [16]. While soluble ACE2 exists after cleavage of ACE2 from the apical cell surface, it plays little to no physiologic role [16].
ACE2 primarily catalyzes the conversion of ANGII into angiotensin- (1-7) (ANG1-7), a metabolite that opposes the actions of ANGII and the RAS axis through activation of the Mas receptor [17]. Generation of ANG1-7 can take a more circuitous route through ACE2 catalyzation of angiotensin I into angiotensin-(1-9) followed by ACE catalyzation of angiotensin-(1-9) into ANG1-7 [17]. Increased circulating levels of ANG1-7 have been demonstrated to lower blood pressure, improve en- dothelial function, and attenuate the effects of ANGII in spontaneously hypertensive rats [17]. Additionally, ANG1-7 administered to spontane- ously hypertensive rats treated with a nitric oxide synthase inhibitor at- tenuated the inhibitor’s effects on MAP, as well as demonstrated Cardioprotective effects in the setting of global cardiac ischemia [18]. ACE2 and ANG1-7 play an essential physiologic role in vasodilation and regulation of endothelial function in opposition to the effects of ANGII. Fig. 1 summarizes the production of ANGII and ANG1-7, as well as their effects. Imbalance of ACE and ACE2 products has the poten- tial to cause significant dysfunction and has been implicated as playing a role in the pathogenesis of SARS [19]. Downregulation of ACE2 expres- sion has been demonstrated after SARS-CoV pulmonary and myocardial infection [19,20] and is linked to the acute pulmonary injury seen in SARS [19].
COVID-19 symptomology is diverse, including shortness of breath, cough, and fatigue with many cases progressing into pneumonia requir- ing oxygen therapy [21,22]. COVID-19 has also been demonstrated to have detrimental effects on extra-Pulmonary systems, including the heart and systemic vasculature [20-24]. SARS-CoV-2 infection has been linked to increased risk for venous thromboembolism and arterial thrombosis [23]. Several markers of coagulopathy, including thrombo- cytopenia, elevated D-dimer and Fibrin degradation products, elevated PT and PTT, and fibrinolysis shutdown, were associated with increased rates of thromboembolic events and mortality in COVID-19 [23,25-27]. Anticoagulation given to COVID-19 patients with coagulopathy showed improved mortality whereas patients with non-COVID-19 disease with similar coagulopathy measurements did not have improved survival [27]. This serves to further implicate coagulation defects as a cause of death in COVID-19, though the underlying mechanism has yet to be confirmed.
Given the similar clinical presentation of COVID-19 and SARS, as well
as use of the same receptor for viral entry, patient data collected during the SARS epidemic provides a framework to begin studying SARS-CoV-2 mechanisms of pathogenesis. Endothelial dysfunction has been

Fig. 1. An illustration of the pathway leading from ANGI to ANGII and ANG1-7, as well as their receptor-mediated effects. Bold arrows indicate the major pathway toward metabolite accumulation and their effects. ANGII production leads to increased blood pressure and endothelial dysfunction, while ANG1-7 production leads to decreased blood pressure and improved endothelial function. Abbreviation: ACE - angiotensin converting enzyme, ACE2 - angiotensin converting enzyme 2, ANGII - angiotensin II, ANG1-7 - angiotensin-(1-7), AT2-R - angiotensin II receptor.
considered to likely contribute to the coagulation abnormalities seen in COVID-19. Post-mortem studies of SARS patients have demonstrated systemic polyangiitis, vasculitis, and infiltration of inflammatory cells with accompanying thrombi in the microcirculation of several organ systems, including the lungs and kidneys [28,29]. An autopsy series of 3 COVID-19 patients indicated systemic viral infection of the endothe- lium with inflammatory cells associated with virally infected cells [30]. The vascular endothelium has come to be seen as an integral participant in the regulation of vascular homeostasis. Endothelial dysfunction is strongly associated with a pro-coagulant state [31]. The inherent tro- pism of SARS-CoV-2 for ACE2-expressing tissues, such as the vascular endothelium, raises the possibility that systemic viral infection of the vascular endothelium is a strong contributing factor to COVID-19- related thromboembolism.
Inflammatory and immune effects further compound endothelial dysfunction in the creation of a pro-coagulant state in COVID-19. Im- mune cell infiltration of virally-infected tissue is evident in both SARS- CoV and SARS-CoV-2 infection [20,28-30]. Immunity and coagulation systems are deeply intertwined, and activation of the immune system during infection will invariably result in a lower threshold for the for- mation of thrombi [32]. Clinical features of SARS-CoV-2 include in- creased production of several inflammatory cytokines that are able to predispose thrombus formation, and infiltration of inflammatory cells into the vascular endothelium may lead to endothelial and platelet acti- vation, further increasing the risk of thromboembolic events [22,32]. Antibody responses may also play a role in COVID-19-related coagula- tion. A series of patients with multiple cerebral infarctions with markers of coagulopathy demonstrated production of antiphospholipid antibod- ies after SARS-CoV-2 infection [33]. Another COVID-19 patient devel- oped immune thrombocytopenic purpura after heparin treatment was begun [34]. Heparin treatment was ceased after thrombocytopenia de- veloped, though Antibody testing for antiplatelet factor 4 and antiplate- let antibodies was negative [34]. These events suggest SARS-CoV-2
Cerebrovascular events in COVID-19″>infection as the precipitating event for the thrombocytopenia in this case, though causative studies are necessary.
The complement system has also been implicated in the pathophys- iology of SARS-CoV-2 infection. A series of patients with severe COVID- 19 were determined to have significant deposits of terminal comple- ment proteins and signs of systemic complement activation were pres- ent [35]. Complement was also co-localized with SARS-CoV-2 S-protein in these patients, indicating complement targeting of virally-infected endothelium [35]. Previous studies of complement activation in SARS- CoV infection indicated endothelial dysfunction as the source of com- plement activation, and murine C3 protein knockout models demon- strated less severe infection with SARS-CoV [36]. Activation of the complement system has the potential to increase the risk of thrombus formation, both through C3a stimulation of platelets and insertion of terminal complement components into membranes [37].
Imbalance of the interactions between ACE2 and the RAS axis may also contribute to the thromboembolic events seen in SARS-CoV-2 in- fection. ANG1-7, the major product of ACE2, and ANGII have competing effects on blood pressure and endothelial activation: where ANGII serves to increase blood pressure and activate the endothelium, ANG1-7 reverses these actions through the Mas receptor (Fig. 1) [17,38]. ANG1-7 has also been shown to decrease thrombus formation
through the production of nitric oxide and prostacyclin by both platelets and endothelial cells [39-41]. This is contrasted by the actions of ANGII, which has been shown to accelerate thrombus formation through in- duction of tissue factor production and generation of free radicals that scavenge free nitric oxide [42,43]. Downregulation of ACE2 by SARS- CoV-2 infection [20] may result in an imbalance of these systems, lead- ing to predisposition to thromboembolic events.
-
- Cerebrovascular events in COVID-19
Cerebrovascular events can be a significant consequence of uncon- trolled thrombotic states, and represent a global burden to both quality of life and national economics [44,45]. Ischemic stroke due to occlusion of large arteries has been a documented complication of SARS-CoV in- fection in patients with minimal to no risk factors [46]. SARS-CoV-2 in- fection seems to also increase risk of developing ischemic stroke, among other neurological consequences. 78 of 214 patients in a retrospective case series of hospitalized patients in Wuhan, China demonstrated ner- vous system dysfunction (CNS, Peripheral nervous system, and/or skel- etal Muscle dysfunction) [47]. Of these 78 patients, 6 developed ischemic strokes; 5 of these patients had been categorized as severe COVID-19 and 1 had been categorized as non-severe [47]. Unexplained
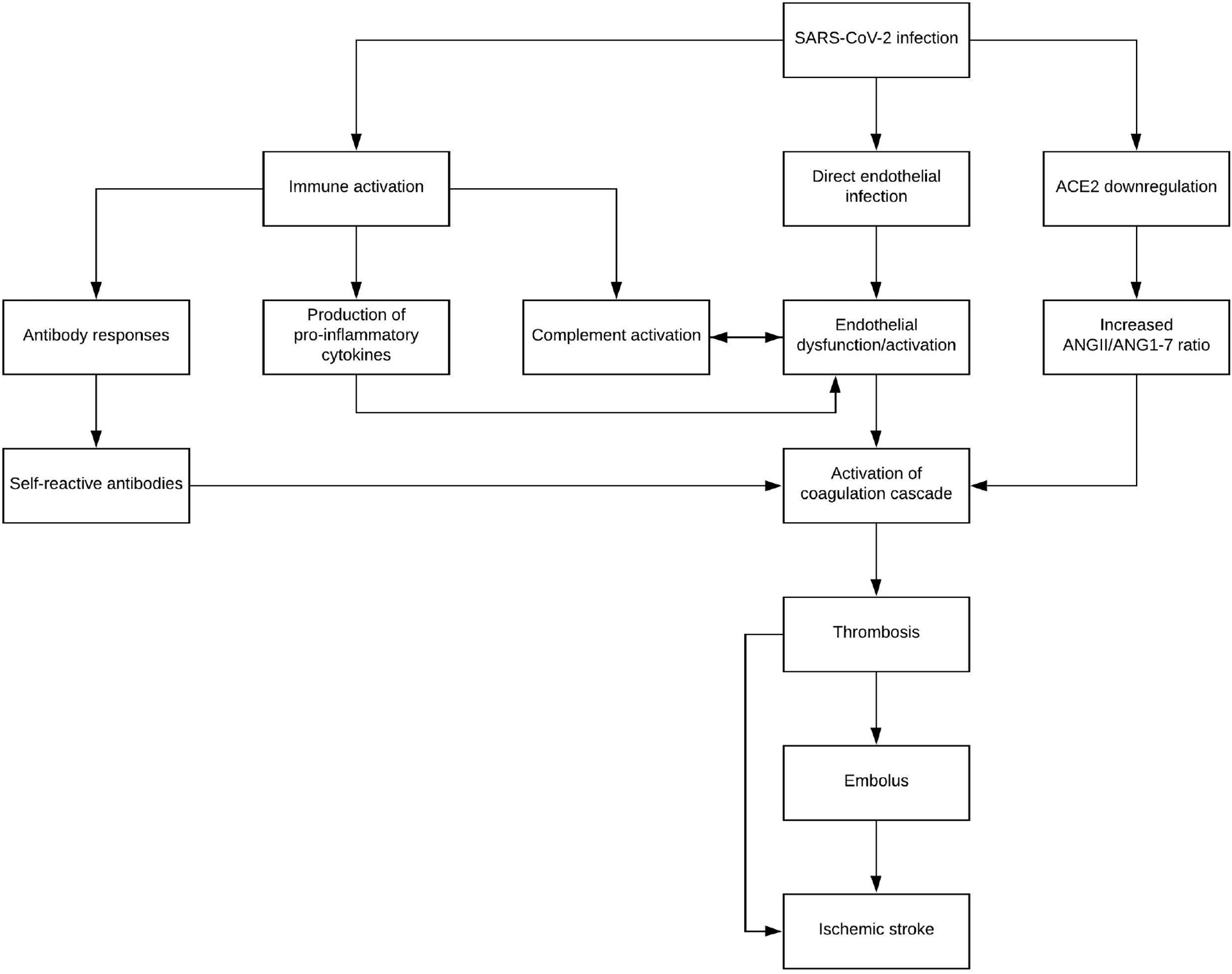
Fig. 2. A theoretical pathway beginning from SARS-CoV-2 to the activation of the coagulation cascade through several mechanisms, including immune activation, direct endothelial infection, and downregulation of ACE2. Hypercoagulable states then lead to ischemic stroke through both embolus formation and thrombosis formation in-situ. Abbreviations: ANGII - angiotensin II, ANG1-7 - angiotensin-(1-7).
encephalopathic features in 13 of 58 patients were seen in another case series of COVID-19 patients [48]. Ischemic stroke was diagnosed in 3 of these 13 patients (2 small acute and 1 sub-acute strokes) [48]. A 40- year-old woman without significant medical history diagnosed with se- vere COVID-19 pneumonia was noted to develop significant left Middle cerebral artery territory stroke after ICU hospitalization [49].
Concerningly, severe respiratory disease is not necessarily requisite to the development of cerebral ischemia. In a retrospective case review at NYU Langone Health, 17 patients undergoing neurologic imaging (CT, MRI, angiography) for reasons unrelated to COVID-19 tested positive for SARS-CoV-2 infection; 4 of these 17 patients had been presenting for symptoms of stroke [50]. Further, stroke has been a presenting or com- plicating factor in mild COVID-19 disease of young patients. Separate case series involving a total of 11 COVID-19 patients all under 55- years-old with minimal Respiratory involvement developed large vessel ischemic stroke [51,52]. A 33-year-old male presenting to the ED for oc- cipital headache, nausea/vomiting, and balance disorder was discovered to have thrombosis of his left vertebral artery with concomitant SARS- CoV-2 infection [53]. A 52-year-old male with history of hypertension was discharged from the ED after being given antibiotics for a presumed respiratory infection before returning days later for stroke symptoms caused by occlusion of the left Internal carotid artery [54].
- Conclusions
While hypertension is known to produce a prothrombotic state [55], lack of many typical stroke risk factors seems to indicate a need for sur- veillance of stroke in COVID-19 patients, as well as the consideration of SARS-CoV-2 infection in patients presenting with cerebrovascular events. Elevated D-dimer, as well as other markers of coagulopathy, has been noted as a potential prognostic factor for COVID-19 [23,25- 27], and these series of case reports provide anecdotal evidence further supporting the use of D-dimer [47,49,51-54]. Ischemic stroke is an un- common, though severe, complication of SARS-CoV-2 infection, and Fig. 2 details the theoretical progression and contributing factors lead- ing from viral infection to stroke. Thrombus formation is a risk that can- not be completely ruled out in COVID-19 patients given the prothrombotic milieu precipitated by this viral syndrome. Whether as a result of embolization of distant thrombus or thrombus formation in situ, ischemic stroke in the setting of COVID-19 is a significant sequalae that warrants further research on its pathophysiologic origins, as well as clinical consideration in patients of diverse demographic origins and risk factor profiles.
This research did not receive any specific grant from funding agen- cies in the public, commercial, or not-for-profit sectors.
No support was used in the creation of this review.
Declaration of Competing Interest
- Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490-502. https://doi.org/10.1016/j. tim.2016.03.003.
- Chan-Yeung M, Xu R. SARS: epidemiology. Respirology. 2003;8(Suppl. 1):S9-S14. https://doi.org/10.1046/j.1440-1843.2003.00518.x.
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727-33. https://doi.org/10.1056/ NEJMoa2001017.
- Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel co- ronavirus: implications for virus origins and receptor binding. Lancet. 2020;395 (10224):565-74. https://doi.org/10.1016/S0140-6736(20)30251-8.
- Liu Y, Gayle AA, Wilder-Smith A, Rocklov J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27(2). https://doi.org/10. 1093/jtm/taaa021.
- Liu T, Hu J, Kang M, et al. Transmission dynamics of 2019 novel coronavirus (2019- NCoV). Soc Sci Res Netw. 2020. https://doi.org/10.2139/ssrn.3526307.
- CDC. Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Preven-
tion; 2020 Published June 17, 2020. Accessed July 10, 2020 https://www.cdc.gov/ coronavirus/2019-ncov/cases-updates/us-cases-deaths.html.
- WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-
opening-remarks-at-the-media-briefing-on-covid-19-11-march-2020; 2020.
[Accessed 10 July 2020].- Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogene- sis. Coronaviruses. 2015;1282:1-23. https://doi.org/10.1007/978-1-4939-2438-7_1.
- Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel corona- virus from Wuhan: an analysis based on decade-long structural studies of SARS co- ronavirus. J Virol. 2020;94(7). https://doi.org/10.1128/JVI.00127-20.
- Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new co- ronavirus of probable bat origin. Nature. 2020;579(7798):270-3. https://doi.org/10. 1038/s41586-020-2012-7.
- Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281-292. e6. https://doi.org/10.1016/j.cell.2020.02.058.
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052.
- Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin-converting enzyme cloning and Functional expression as a captopril- insensitive carboxypeptidase. J Biol Chem. 2000;275(43):33238-43. https://doi. org/10.1074/jbc.M002615200.
- Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532 (1-2):107-10. https://doi.org/10.1016/S0014-5793(02)03640-2.
- Hamming I, Timens W, Bulthuis M, Lely A, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in under- standing SARS pathogenesis. J Pathol. 2004;203(2):631-7. https://doi.org/10.1002/ path.1570.
- Brit Rentzsch, Mihail Todiras, Radu Iliescu, et al. Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and im- proves endothelial function. Hypertension. 2008;52(5):967-73. https://doi.org/10. 1161/HYPERTENSIONAHA.108.114322.
- Benter IF, Yousif MHM, Anim JT, Cojocel C, Diz DI. Angiotensin-(1-7) prevents devel- opment of Severe hypertension and end-organ damage in spontaneously hyperten- sive rats treated with l-NAME. Am J Physiol Heart Circ Physiol. 2006;290(2): H684-91. https://doi.org/10.1152/ajpheart.00632.2005.
- Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11(8):875-9. https://doi. org/10.1038/nm1267.
- Oudit GY, Kassiri Z, Jiang C, et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39(7): 618-25. https://doi.org/10.1111/j.1365-2362.2009.02153.x.
- Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708-20. https://doi.org/10.1056/ NEJMoa2002032.
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. https://doi.org/ 10.1016/S0140-6736(20)30183-5.
- Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-7. https:// doi.org/10.1016/j.thromres.2020.04.013.
- Marone EM, Rinaldi LF. Upsurge of deep venous thrombosis in patients affected by COVID-19: preliminary data and possible explanations. J Vasc Surg Venous Lymphat Disord. 2020;8(4):694-5. https://doi.org/10.1016/j.jvsv.2020.04.004.
- Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-7. https://doi.org/10.1111/jth.14768.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpa- tients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020; 395(10229):1054-62. https://doi.org/10.1016/S0140-6736(20)30566-3.
- Wright FL, Vogler TO, Moore EE, et al. Fibrinolysis shutdown correlation with throm- boembolic events in severe COVID-19 infection. J Am Coll Surg. 2020. https://doi. org/10.1016/j.jamcollsurg.2020.05.007 Published online May 15.
- Xiang-hua Y, Le-min W, Ai-bin L, et al. Severe acute respiratory syndrome and ve- nous thromboembolism in multiple organs. Am J Respir Crit Care Med. 2010;182
(3):436-7. https://doi.org/10.1164/ajrccm.182.3.436.
- Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syn- drome (SARS): a report from China. J Pathol. 2003;200(3):282-9. https://doi.org/10. 1002/path.1440.
- Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417-8. https://doi.org/10.1016/S0140-6736 (20)30937-5.
- Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: a marker of atheroscle- rotic risk. Arterioscler Thromb Vasc Biol. 2003;23(2):168-75. https://doi.org/10. 1161/01.atv.0000051384.43104.fc.
- Esmon CT. Interactions between the innate immune and blood coagulation systems. Trends Immunol. 2004;25(10):536-42. https://doi.org/10.1016/j.it.2004.08.003.
- Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and Antiphospholipid antibodies in patients with Covid-19. N Engl J Med. April 8, 2020. https://doi.org/10.1056/ NEJMc2007575 Published online.
- Zulfiqar A-A, Lorenzo-Villalba N, Hassler P, Andres E. Immune thrombocytopenic Purpura in a patient with Covid-19. N Engl J Med. April 15, 2020. https://doi.org/ 10.1056/NEJMc2010472 Published online.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microVascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13. https://doi.org/10.1016/j.trsl.2020.04.007.
- Campbell CM, Kahwash R. Will complement inhibition be the new target in treating COVID-19-related systemic thrombosis? Circulation. 2020;141(22):1739-41. https://doi.org/10.1161/CIRCULATIONAHA.120.047419.
- Ruf W. Links between complement activation and thrombosis. Blood. 2019;134 (Supplement_1):SCI-SCI-40. https://doi.org/10.1182/blood-2019-121113.
- Santos RAS, Sampaio WO, Alzamora AC, et al. The ACE2/angiotensin-(1-7)/MAS Axis of the renin-angiotensin system: focus on angiotensin-(1-7). Physiol Rev. 2018;98 (1):505-53. https://doi.org/10.1152/physrev.00023.2016.
- Fang C, Stavrou E, Schmaier AA, et al. Angiotensin 1-7 and mas decrease thrombosis in Bdkrb2-/- mice by increasing NO and prostacyclin to reduce platelet spreading and glycoprotein VI activation. Blood. 2013;121(15):3023-32. https://doi.org/10. 1182/blood-2012-09-459156.
- Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, et al. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect. Clinics (Sao Paulo). 2011;66 (5):837-41. https://doi.org/10.1590/S1807-59322011000500021.
- Fraga-Silva RA, Pinheiro SVB, Goncalves ACC, Alenina N, Bader M, Santos RAS. The antithrombotic effect of angiotensin-(1-7) involves mas-mediated NO release from platelets. Mol Med. 2008;14(1-2):28-35. https://doi.org/10.2119/2007- 00073.Fraga-Silva.
- Senchenkova EY, Russell J, Esmon CT, Granger DN. Roles of coagulation and fibrino- lysis in angiotensin II enhanced microvascular thrombosis. Microcirculation. 2014; 21(5):401-7. https://doi.org/10.1111/micc.12120.
- Brown NJ, Vaughan DE. Prothrombotic effects of angiotensin. Adv Intern Med. 2000; 45:419-29.
- Mukherjee D, Patil CG. Epidemiology and the global burden of stroke. World
Neurosurg. 2011;76(6 Suppl):S85-90. https://doi.org/10.1016/j.wneu.2011.07.023.
- Di Carlo A. Human and Economic burden of stroke. Age Ageing. 2009;38(1):4-5. https://doi.org/10.1093/ageing/afn282.
- Umapathi T, Kor AC, Venketasubramanian N, et al. Large artery ischaemic stroke in Severe acute respiratory syndrome . J Neurol. 2004;251(10):1227-31. https://doi.org/10.1007/s00415-004-0519-8.
- Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1-9. https:// doi.org/10.1001/jamaneurol.2020.1127.
- Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infec- tion. N Engl J Med. April 15, 2020. https://doi.org/10.1056/NEJMc2008597 Published online.
- Gunasekaran K, Amoah K, Rajasurya V, Buscher MG. Stroke in a young COVID-19 patient. QJM. May 22, 2020. https://doi.org/10.1093/qjmed/hcaa177 Published online.
- Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Raz E. Surprise diagnosis of COVID- 19 following neuroimaging evaluation for unrelated reasons during the pandemic in hot spots. Am J Neuroradiol. May 28, 2020. https://doi.org/10.3174/ajnr.A6608 Pub- lished online.
- Ashrafi F, Zali A, Ommi D, et al. COVID-19-related strokes in adults below 55 years of age: a case series. Neurol Sci. June 24, 2020:1-5. https://doi.org/10.1007/s10072- 020-04521-3 Published online.
- Oxley TJ, Mocco J, Majidi S, et al. Large-vessel stroke as a presenting feature of Covid-
19 in the young. N Engl J Med. April 28, 2020. https://doi.org/10.1056/ NEJMc2009787 Published online.
- Cavallieri F, Marti A, Fasano A, et al. Prothrombotic state induced by COVID-19 infec- tion as trigger for stroke in young patients: a dangerous association. eNeurological Sci. 2020;20. https://doi.org/10.1016/j.ensci.2020.100247.
- Valdes Valderrama Eduard, Kelley Humbert, Aaron Lord, Jennifer Frontera, Shadi Yaghi. severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. 2020;51(7):e124-7. https://doi.org/10.1161/STROKEAHA.120. 030153.
- Nadar S, Lip GYH. The prothrombotic state in hypertension and the effects of antihy- pertensive treatment. Curr Pharm Des. 2003;9(21):1715-32. https://doi.org/10. 2174/1381612033454559.
