Hypoalbuminaemia predicts clinical outcome in patients with type B acute aortic dissection following endovascular therapy
Affiliations
- Department of Cardiology, West China Hospital, Sichuan University, Chengdu, China
Affiliations
- Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, China
Affiliations
- Department of Cardiology, West China Hospital, Sichuan University, Chengdu, China
Affiliations
- Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, China
Affiliations
- Department of Cardiology, West China Hospital, Sichuan University, Chengdu, China
Affiliations
- Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, China
Affiliations
- Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, China
Affiliations
- Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, China
Correspondence
- Corresponding authors at: Department of Emergency Medicine, West China, Hospital, Sichuan University, 37 Guoxue Road, Chengdu, 610041, Sichuan, China. Tel./fax: +86 28 83584358.

Affiliations
- Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, China
Correspondence
- Corresponding authors at: Department of Emergency Medicine, West China, Hospital, Sichuan University, 37 Guoxue Road, Chengdu, 610041, Sichuan, China. Tel./fax: +86 28 83584358.
Affiliations
- Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, China
Correspondence
- Corresponding authors at: Department of Emergency Medicine, West China, Hospital, Sichuan University, 37 Guoxue Road, Chengdu, 610041, Sichuan, China. Tel./fax: +86 28 83584358.

Affiliations
- Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, China
Correspondence
- Corresponding authors at: Department of Emergency Medicine, West China, Hospital, Sichuan University, 37 Guoxue Road, Chengdu, 610041, Sichuan, China. Tel./fax: +86 28 83584358.
Affiliations
- Department of Cardiology, West China Hospital, Sichuan University, Chengdu, China
- Department of Emergency Medicine, West China Hospital, Sichuan University, Chengdu, China
 Article Info
Article Info
To view the full text, please login as a subscribed user or purchase a subscription. Click here to view the full text on ScienceDirect.
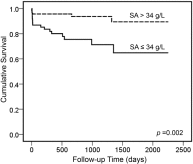
Fig. 1
Kaplan–Meier survival analysis showing patients with type B acute aortic dissection with hypoalbuminaemia (serum albumin [SA] ≤ 34 g/L) had significantly worse survival than patients without hypoalbuminaemia.
Abstract
Background
Few studies have reported that serum albumin (SA) levels on admission were associated with increased risk of long-term outcomes in patients with type B acute aortic dissection (AAD). The aim of this study was to investigate the effect of admission levels of SA on survival among patients with type B AAD undergoing endovascular therapy (EVT).
Methods
A total of 131 patients with type B AAD undergoing EVT were retrospectively enrolled and followed up for 2.1 years. They were divided into hypoalbuminemia and non-hypoalbuminemia groups. We analyzed the incidence of in-hospital complications and long-term mortality. Kaplan–Meier curves and multivariable Cox regression analyses were used to investigate the associations between SA levels and survival.
Results
Among 131 type B AAD patients, hypoalbuminemia was detected in 61 (46.6%) at admission. Compared to those without hypoalbuminemia, patients with hypoalbuminemia did not have higher in-hospital complications; however, Kaplan–Meier analysis showed that they did have a significantly lower survival rate (73.8% vs. 92.5%, log-rank χ2 = 9.8, P = .002). Multivariable Cox regression analysis further revealed that hypoalbuminemia was an independent predictor of long-term mortality among patients with type B AAD (hazard ratio, 4.28; 95% confidence interval, 1.36–13.47; P = .013), over 2.1 years.
Conclusions
Hypoalbuminemia is common in type B AAD patients and is independently associated with increased risk of long-term death. Renal dysfunction may be the main pathophysiological mechanism underlying hypoalbuminemia in patients with type B AAD.
Abbreviations:
SA (serum albumin), AAD (acute aortic dissection), EVT (endovascular therapy), CRP (C-reactive protein), Cr (creatinine), eGFR (estimated glomerular filtrate rate), Hb (hemoglobin), SBP (systolic blood pressure at admission), DBP (diastolic blood pressure at admission), LVEF (left ventricular ejection fraction), BUN (blood urea nitrogen), ALT (alanine aminotransferase), AST (aspartate aminotransferase), TFL (thrombosed false lumen), ARB (angiotensin II receptor), ACEI (angiotensin-converting enzyme inhibitor), CCB (calcium-channel blocker.), MI (myocardial infarction or ischemia), HR (hazard ratio), CI (confidence interval)To access this article, please choose from the options below
Purchase access to this article
Claim Access
If you are a current subscriber with Society Membership or an Account Number, claim your access now.
Subscribe to this title
Purchase a subscription to gain access to this and all other articles in this journal.
Institutional Access
Visit ScienceDirect to see if you have access via your institution.
Related Articles
Searching for related articles..


